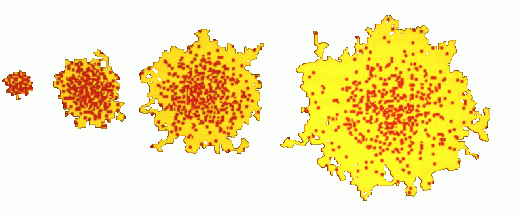
1. Conceptual Model of Diffusion

This section introduces the concept of molecular diffusion based on the random path of molecules. The theory and animation demonstrate how, through Brownian motion, a Gaussian distribution of molecules is obtained from a point release. The diffusion coefficient, D, is introduced as the coefficient in Fick's Law - which states that mass flux is directly proportional to, and in the opposite direction of, the concentration gradient. As noted by Fischer et al. (1979), several environmental dispersion problems can be described by processes that are strongly analagous to molecular diffusion, so it's an incredibly important concept! The example problems test one's knowledge of Fick's Law, the physical meaning of the diffusion coefficient, and the use of the Gaussian distribution to describe concentration profiles.
(these links open in new browser windows)