V
APOR FRACTION
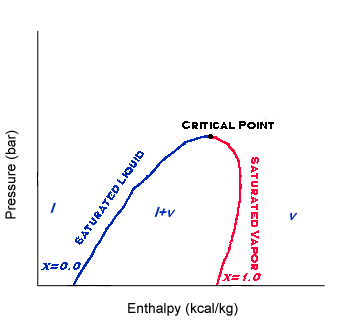 When a compound is in
the liquid-vapor dome, it is often useful to know what
fraction of the compound is in a saturated liquid state and what fraction is in a
saturated vapor state. The term, vapor fraction, gives the fraction that is in a
gaseous state. Vapor fraction is represented by the variable, x.
When a compound is in
the liquid-vapor dome, it is often useful to know what
fraction of the compound is in a saturated liquid state and what fraction is in a
saturated vapor state. The term, vapor fraction, gives the fraction that is in a
gaseous state. Vapor fraction is represented by the variable, x.
The line left of the critical point on the dome is the saturated
liquid line. That means the vapor fraction anywhere on that line is 0. However, the line
to the right on the dome is the saturated vapor line. That means the vapor fraction on
that line is 1. Any point between those too lines then has a vapor fraction value between
0 and 1.
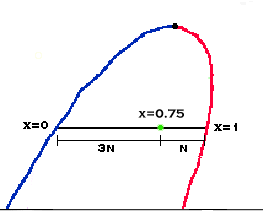
The relation between position in the dome and vapor fraction is
linear. That is, a point halfway between the saturated vapor line and the saturated
liquid line has a vapor fraction of 0.5. A point (see diagram) that is 3 times farther
from the liquid line then the vapor line has a vapor fraction of 0.75. This kind of
calculation follows the lever rule.
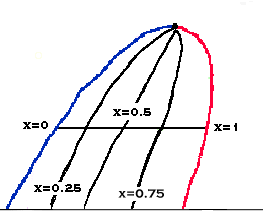
We can then draw a line between all points that have the same
vapor fraction to give a Constant x line. Note that the saturated vapor line is a
Constant x line with x=1, and a saturated liquid line is a Constant x line with x=0.
Previous: Liquid-Vapor Dome ------- Next: The Lever Rule
 When a compound is in
the liquid-vapor dome, it is often useful to know what
fraction of the compound is in a saturated liquid state and what fraction is in a
saturated vapor state. The term, vapor fraction, gives the fraction that is in a
gaseous state. Vapor fraction is represented by the variable, x.
When a compound is in
the liquid-vapor dome, it is often useful to know what
fraction of the compound is in a saturated liquid state and what fraction is in a
saturated vapor state. The term, vapor fraction, gives the fraction that is in a
gaseous state. Vapor fraction is represented by the variable, x.
