VOLUME (intensive)
units=cm^3/mol
grey=lines of constant volume
![]()
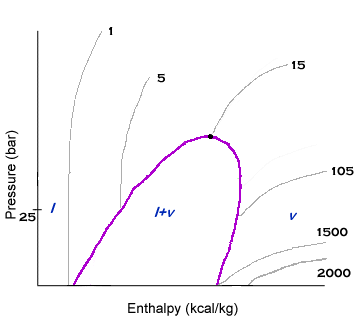
Volume lines are upward sloping lines. The volume lines are usually flatter in slope than entropy lines. However, at lower enthalpies, the volume lines become steeper. The volume values will not change that much from line to line at lower enthalpies. This is because liquids and solids are relatively incompressible when compared to vapor. In the liquid-vapor dome, the volume lines are usually not listed.
Instead of reading a diagram, equations of state [EOS] (ideal gas law, cubic EOS, etc.) can be used to calculate volume. Other values and slopes can also be calculated by the EOS and Cp data.