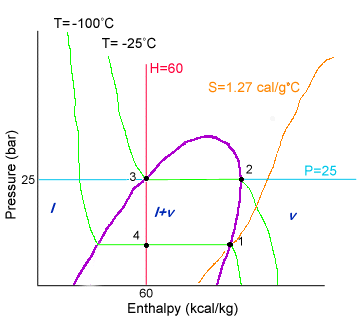
Pressure-Enthalpy Diagram for Ethylene

So how did we get these points?
Points 1 and 3:
From the given information in the table, we know that 1 is saturated vapor at -100°C, and
3 is saturated liquid at -25°C. Saturated liquids and vapors are on the liquid-vapor dome
(shown in purple). To find points 3 and 1, we follow the isotherms for -100 and -25 (shown
in green).
Point 4:
From the given information, P4=P1 and it is a mixture of saturated liquid and saturated
vapor. Since it is both liquid and vapor, that places the point inside the liquid-vapor
dome. Since P4=P1, 4 lies on the same horizontal line as 1. This still does not give an
exact point. However, from our energy balance around the valve, we discovered that H3=H4.
H3 is at 60, so point 4 lies on the intersection of the isobaric line with the enthalpy=60
line (shown in red).
Point 2:
At 2, we know that P2=P3 and S2=S1 from the given information. The intersection of the
isobaric line with the entropy=1.27 line (shown in orange) gives point 2.
The table can now be filled out by reading the diagram as follows:
| stream i | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| phase(s) | sat. vapor | vapor | sat. liquid | sat. liquid & saturated vapor |
| initial temp (°C) |
-100 | 50 | -25 | -100 |
| initial pressure (bar) |
1.3 | P2=P3 | 25 | P4=P1 |
| H (kcal/kg) |
120 | 175 | 60 | 5 liquid 120 vapor |
| S (cal/g°C) |
1.27 | S2=S1 | X | X |
Notice that the enthalpies for 4 correspond to the enthalpies for the saturated liquid and the saturated vapor at -100.