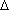 Ut.
Ut.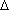 Ht.
Ht.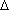 H.
H.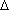 Ht=Q.
Ht=Q.Problem 2 (Due Monday, September 13)
A piston contains 1 kg of argon gas at 300 K and 1 bar. The initial volume occupied by the gas is 0.624 m3. Heat, in the amount of 104.2 kJ, is added to elevate the temperature of the argon to 500 K while the pressure in the piston remains constant. The final gas volume is 1.041 m3.
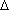 Ut.
Ut.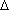 Ht.
Ht.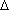 H.
H.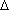 Ht=Q.
Ht=Q.