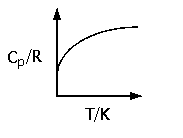
The empirical form for the temperature dependence of Cp is
Cp/R = A + BT + CT2 + D/T2 ,
where the constants A, B, C and D can be found for various gases in Table 4.1 (pg. 109) of S&VN. Depending on the temperature range of interest, this variation can be significant (see Fig. 4.1 of S&VN, pg. 108).

| FOR ENTHALPY | FOR ENTROPY | |
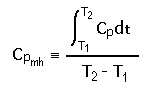 |
|
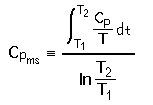 |
Substituting for Cp and integrating, we find
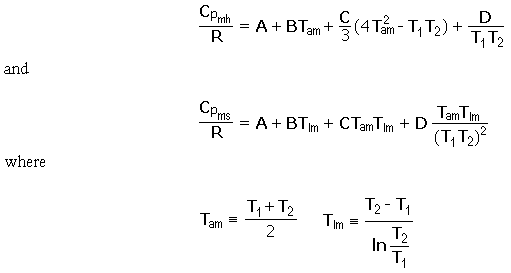
Enthalpy and entropy changes at constant pressure are then calculated for 1 mol by
