10.491: Integrated Chemical Engineering
Prof. Robert Langer
Problem Set 2
May 1, 2000
Problem:
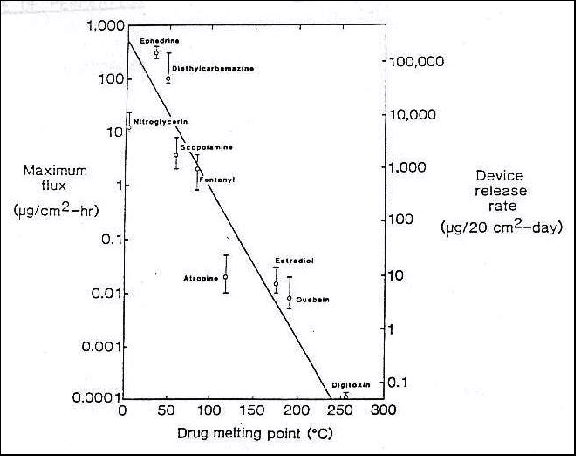
Data has shown that the flux of drug from a
transdermal delivery system decreases logarithmically as the melting
temperature of the enclosed drug increases. Is there an explanation for this,
and if so, what is it? Hint: Consider the thermodynamics of the system.
Solution:
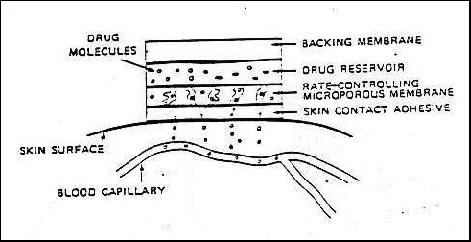
A schematic of a typical transdermal drug delivery system
is shown in the figure below. The drug is loaded into a reservoir, most often in
a supersaturated solution to maintain a constant dissolved drug concentration,
from which it diffuses through a polymeric membrane and the patient’s skin. To
decrease the influence of patient-to-patient variability, the device is
designed such that the diffusion through the polymer is the rate-limiting step.
Because the drug is assumed to nearly instantly disperse upon penetration into
the blood stream, the internal concentration of drug is considered to be
negligible and the body is treated as an “infinite sink.”
![]()
The general form of
the equation describing flux in the a system with supersaturated drug and an
infinite sink is
where F is flux, D is the diffusivity
constant, K is the partition coefficient, CS is the
drug solubility, A is the surface area, and L is the length
through which the drug must diffuse. Of these parameters, D, K,
and CS will be affected by the properties of the drug, and CS
will be influenced the most strongly by the drug's melting temperature. The
ideal law of solubility gives the relationship between solubility and melting
temperature. What follows is a description of the derivation of this
relationship.
![]()
If we assume that the
drug is present in a supersaturated dispersion in the device, then the solid
and liquid drug must be in chemical equilibrium with one another with
![]()
This assumes that the
solid is pure drug and the liquid has a mole fraction xD of
drug. If we also assume that the liquid solution behaves ideally, then from the
definition of an ideal solution we have
with μLo signifying
the chemical potential of the pure liquid drug. Combining (2) and (3) gives
![]()
![]()
From the definition of the Gibbs energy of fusion of a pure liquid at temperature T and pressure P,
![]() (5)
(5)
Combining
(4) and (5) gives
![]()
Differentiating by xD at constant P
gives
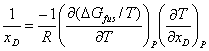 (6)
(6)
From the definition of Gibbs free energy,
![]() (7)
(7)
Differentiating by T at constant P gives
![]() (8)
(8)
From the definition of enthalpy,
![]()
![]() (9)
(9)
From the first law of thermodynamics for closed,
reversible systems,
![]() (10)
(10)
From the definition of reversible mechanical work,
![]() (11)
(11)
From the definition of entropy,
![]() (12)
(12)
Combining (10)-(12) gives
![]() (13)
(13)
Combining (9) and (13) gives
![]()
Taking the partial derivative with respect to T
at constant P gives
![]() (14)
(14)
Combining (8) and (14) gives
![]() (15)
(15)
Also, differentiating G/T
by T at constant P gives
![]() (16)
(16)
Combining (15) and (16) gives
![]() (17)
(17)
Combining (7) and (17) gives
![]() (18)
(18)
This is the Gibbs-Helmholtz equation. Combining (6) and (18) gives
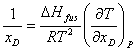
Integrating from the case of pure drug with the
freezing/melting point Tm to drug in solution with
freezing/melting point T, assuming ΔHfus is
constant from Tm to T, gives
![]()
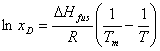
This is the ideal law of solubility. From the equation
it can be seen that as Tm increases, the mole fraction of
drug in solution, xD, decreases logarithmically. Because the concentration
of drug in solution in the device drug reservoir is proportional to xD,
CS will also decrease with increasing Tm. From
equation (1), then, this will result in the logarithmically decreasing trend
seen in the graph of the problem statement.