Summary of Findings
General characteristics:
Rainforest soils are catagorized as oxisols (the red regions of the map). The A horizon, comprised of humus and nutrients, is shallow, thus easily depleted by deforestation. These soils lack a substantial nutrient layer due to extensive rainfall and high temperatures. As a result, the flora has adapted to absorb nutrients directly from decaying plant matter. Rapid bacterial decay makes this possible, as nutrients are released quickly once something begins to decompose. Beneath the A horizon, the thick B horizon consists mostly of clay, sand, and iron and aluminum oxides (giving the soil its red color) that do not retain water well.
Like most soils, tropical soils have a low pH. This causes high concentrations of aluminum and iron. Tropical species can tolerate these high levels of alumunium (whereas it would be toxic to other plants), however, if acidity is increased, the soils can become infertile.
Oxisols also have low ion exchange capacity. That is, ions are not readily available to the plant life. To accommodate this, certain fungi have developed a symbiotic relationship with higher order plants. The fungi, called myccorhizae, dissolves P, Ca, and Mg for the infected plants, while the latter provides the fungus with sugars. If the soil becomes overexposed to sunlight, however, the mycorrhizae die in the baked soil, decreasing the amount of ion absorption by plants, and thus their health and resilience.
pH |
Exchangeable Calcium and Magnesium |
Exchangeable Aluminum |
4.2 |
0.1 meq/100g |
1.0 meq/100g |
4.1 |
0.2 meq/100g |
1.9 meq/100g |
Map of Soil Locations:
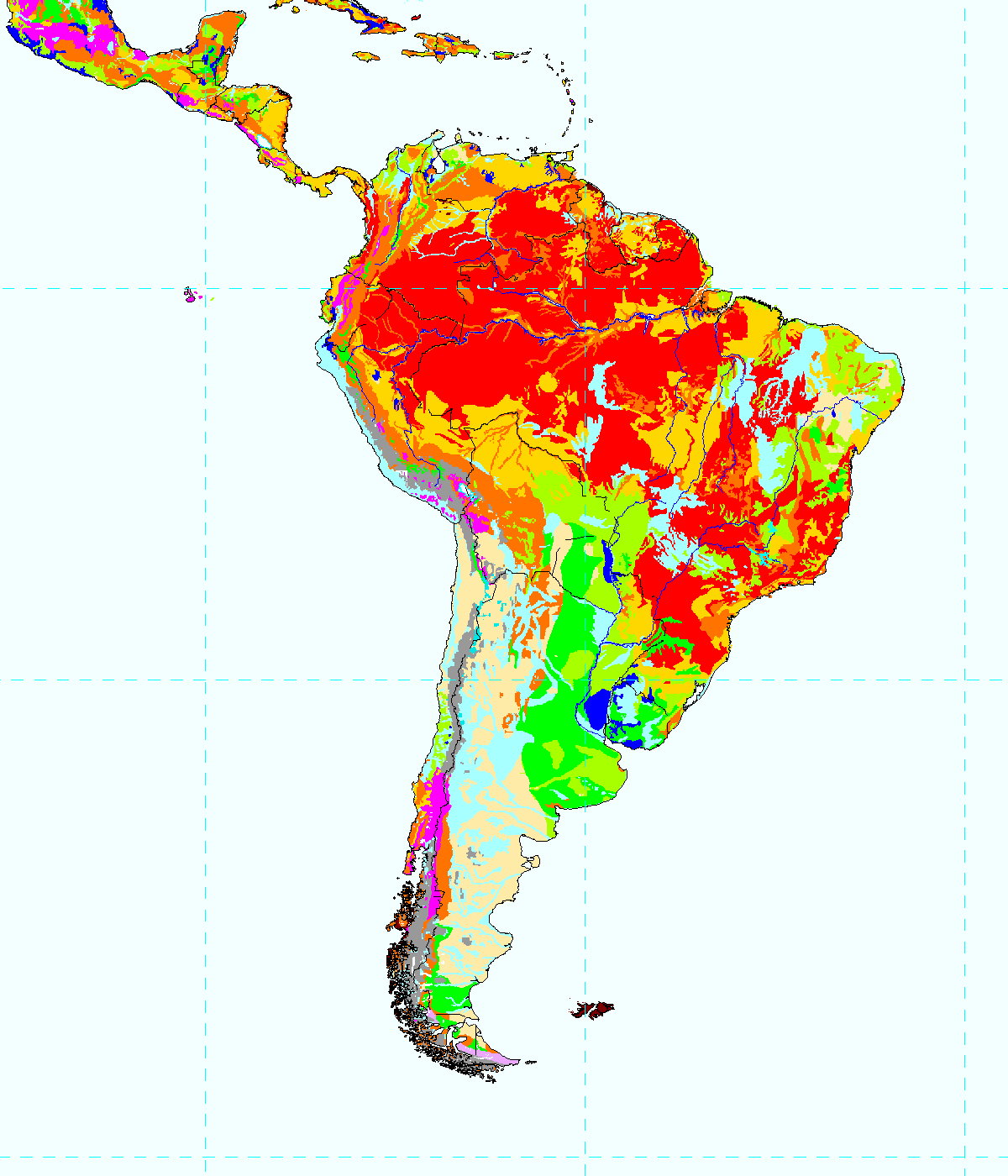
You can see that the Amazon Basin consists mostly of oxisol type soil (described above). Also notice the dark orange areas following the Amazon (and other) rivers. These are Inceptisols, soils highly weathered by water, thus releasing free iron oxides. Through this process, this a clay can form, called allophane, with a high water-holding capacity and incomplete dispersibility. Therefore, this soil traps organic matter, protecting it against rapid decomposition. If leaching is prevented, these can make excellent soils.
Nitrogen Cycle:
The nitrogen cycle is the movement of nitrogen throughout a system. First, gaseous nitrogen reacts with oxygen in the atmosphere to form nitrates, which are carried to the Earth’s surface through precipitation. Once in the soil, these nitrates are “fixed”, meaning they are taken up by plants and incorporated into plant tissues. The nitrates are passed through the food chain. When plants or animals die, their decaying remains release nitrogenous compounds into the soil that decompose into ammonia (a process called “ammoniafication”). This ammonia can then be absorbed directly by plants, leached away by water, or converted into nitrates by microorganisms (called “nitrification”). Some nitrogen is lost back into the atmosphere as free nitrogen through “dentrification”. Aside from natural nitrogen loss, agriculture depleats soils of nitrogen, often requiring the use of fertilizers (which are mostly nitrogen). This sometimes helps the soil quality, however, this excess nitrogen also leaches into the surrounding water systems, decreasing the water quality.
Two main processes govern nitrogen availability in the soil. The first is the addition of nitrogen to the soil through decomposition of organic matter (in the form of ammonia). The second is the conversion of this ammonia into an inorganic form (nitrates), which makes it more available to local vegetation. For soil to be in equilibrium, the rate of addition (conversion into nitrates) should equal the rate of decomposition (decaying plant matter).
Carbont Cycle:
Carbon dioxide, found in the atmosphere or dissolved in water is converted into plant tissues through photosynthesis. Not all carbon dioxide is used in this process however, as some is released once again into the atmosphere by aerobic respiration. Herbivores then eat the plants and degrade (digest, decompose) the carbon. Much of this carbon is then given off as CO2, which can then be reused by the plants.
Numerical Data:
Exchangeable Cations in Two Types of Oxisol in the Cerrado Region
of Brazil (characterization)
(Mendonca, Eduardo S. & Rowell, David L., 1996)
Yellow Red Latolsols
Horizon |
pH |
Ca2+ |
Mg2+ |
K+ |
Na+ |
Al3+ |
Effective Cation Exchange Capacity |
A1 |
5.11 |
0.05 |
0.05 |
0.16 |
0.02 |
0.66 |
0.94 |
A3 |
5.16 |
0.03 |
0.03 |
0.11 |
0.03 |
O.23 |
0.43 |
B1 |
5.27 |
0.04 |
0.03 |
0.08 |
0.02 |
0.10 |
0.27 |
B2 |
5.21 |
0.03 |
0.01 |
0.03 |
0.02 |
0.01 |
0.10 |
Dark Red Latolsols
Horizon |
pH |
Ca2+ |
Mg2+ |
K+ |
Na+ |
Al3+ |
Effective Cation Exchange Capacity |
A1 |
5.13 |
0.01 |
0.03 |
0.14 |
0.01 |
0.67 |
0.86 |
A3 |
5.15 |
0.0 |
0.01 |
0.04 |
0.01 |
0.56 |
0.62 |
B1 |
5.21 |
0.0 |
0.01 |
0.03 |
0.01 |
0.53 |
0.58 |
B21 |
5.13 |
0.0 |
0.01 |
0.02 |
0.01 |
0.46 |
0.50 |
B22 |
5.22 |
0.0 |
0.01 |
0.01 |
0.01 |
0.32 |
0.35 |
*Effective Cation Exchange Capacity is measured in milliequivalents per 100g. It is a measure of tha capacity of clays to aobsorb and exchange cations (positively charged ions). Humus has a CEC of 150.
Monitoring:
We have identified pH and ion concentrations to be important
factors in monitoring.
Here is one lab method commonly used:
1. Drying
- soils should be dried as rapidly as possible to minimize microbial activity
- accomplished by exposing as much as surface of the soil to circulate air
as possible and by evaluating the drying temperature*
*not to exceed 38 C to preserve the physiochemical properties of the sample
2. Crushing
- By hand or mechanical (stainless steel)
- Filter through a 2mm screen to remove rocks and crate a uniform sample
3. Divide samples by volume into equal portions
Testing for pH
- use pH meter with two separate electrodes
- prepare a “soil slurry” of 0.01M CaCl2 2H20* in ratio 1:2
*Recommended for sandy soils or soils with low cation exchange. Usually causes
the pH to appear 0.3 to 0.5 lower therefore, take this into account
Testing for ions
Use CaCl2 to indicate the presence of Na, Mg, K ions.
- Add 0.01M CaCl2 to soil sample and shake it for two hours (mechanically).
- Centrifuge and collect the supernatant and analyze for elemental composition.
- Use plasma emission spectrometry (with emission lines, etc.) to determine
percent of Na, Mg, and K ions.