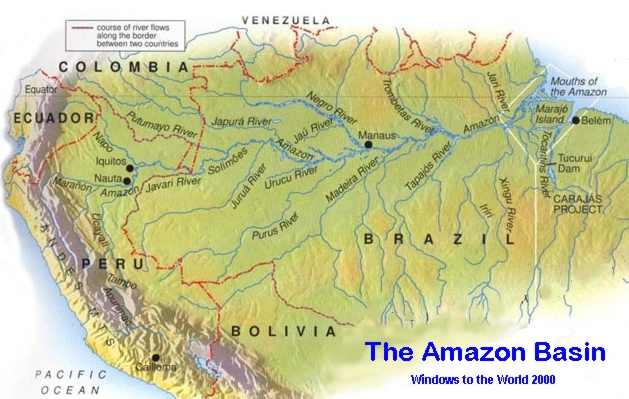
Background: Plants require nitrogen for healthy growth. Most of the nitrogen in organic matter is in a form that plants cannot use. Bacteria found in the siol converts these organic forms of nitrogen into forms that the plant can use. When plants die, they decompose and become part of the organic matter of the soil and the cycle is repeated again.
The nitrogen cycle is one of the most important nutrient cycles found in terrestrial ecosystems. Nitrogen is the building block of many complex molecules formed by plants and animals; some examples are amino acids, proteins, and nucleic acids used in DNA.
Most of the world's nitrogen is located in the atmosphere as N2 gas. Still, atmospheric nitrogen is unuseful to the plants. This is because plants can only consume nitrogen in two solid forms: the ammonium anion (NH4+) and the nitrate anion (NO3-). However, the former is more preferred than the latter because large concentrations of ammonium is extremely toxic. (1)
In most ecosystems, nitrogen is primarily sotred in living and dead organic matter. This nitrogen is converted into inorganic forms (forms usable by plants) when it re- enters the biogeochemical cycle (Cycling of chemicals through the biosphere, lithosphere, hydrosphere, and atmosphere) via decomposition. (2) Decompositoin is achieved by various decomposers found in the upper soil layer. They alter the nitrogen found in organic matter into such forms as ammonium salts. This process, known as mineralization, is carried out by a variety of bacteria, actinomycetes, and fungi. (3)
Animals also require a certain amount of nitrogen in their system for survival. Animals, however, secure their nitrogen fixation through plants or other animals that have fed on plants.
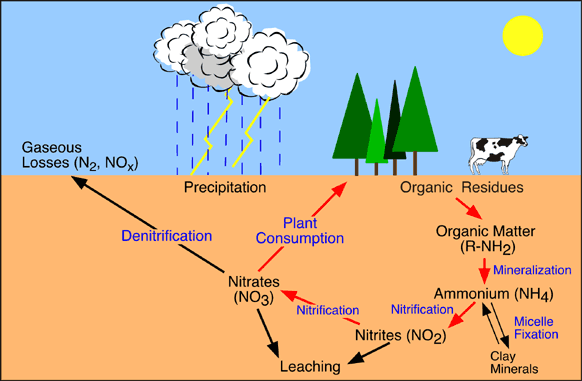
Four processes participate in the cycling of nitrogen through the biosphere: Nitrogen Fixation Decay Nitrification Denitrification (4)
Nitrogen Fixation:The nitrogen molecule (N2) is inert. It is also stable because of the strengh of the tripple bond that it possesses. Thus, breaking it apart will require a substancial amount of energy. Most industrial fixation is achieved under great pressure, at temperatures of 600 degrees celcius, and with the use of a catalyst. Under these conditions, atmospheric nitrogen and hydrogen can be combined to form ammonia. This can be used directly as a fertilizer, however, it is further processed into more useful forms such as urea and ammonium nitrate.
The ability to fix nitrogen is found only in certain bacteria. The first step in this process produces ammonia. However it is quickly incorporated into protein and other organic nitrogen compounds.
Decay:When plants die, they decay and leave behind organic forms of nitrogen. This is changed into more useful inorganic forms by certain bacteria.
Nitrification:Ammonia can be taken up directly by plants- usually through their roots. However, most of the ammonia produced by decay is convertd into nitrates. This is accomplished in two steps: *Bacteria of the genus Nitrosomonas oxidize ammonia to nitrites. *Bacteria of the genus Nitrobacter oxidize nitrites to nitrates. These two groups of bacteria are called nitrifying bacteria. (5) Through their activities, nitrogen is made available to the roots of plants.
Denitrification:The previous processes remove nitrogen from the atmosphere and pass it through the ecosystems. Denitrification reduces nitrates to nitrogen gas, thus replenishing the atmosphere. Denitrification is achieved by bacteria that live deep in the soil and in aquatic sediments. Because the conditions are anaerobic, they use the nitrates as an alternatie to oxigen for the final electron accceptor in their respiration. (6)
Another process, called volatilization turns urea fertilizers and manures on the soil surface into gases that also join the atmosphere. (7) This completes the nitrogen cycle.
This completes the nitrogen cycle.
Most of the human activies responsible for the increase in global nitrogen are local in scale, from the production and use of nitrogen fertilizers to the burning of fossil fuels in automobiles, power generation plants, and industries.
Nitrogen Fertilizers:Industrial fixation of nitrogen for use as fertilizer currently totals approximately 80Tg per year and represents by far the largest human contribution of new nitrogen to the global cycle. This figure does not include manure and other organic nitrogen fertilizers. (8)
The use of fertilizer application in developed countries has stabilized, however, it has risen drastically for developing countries. The momentum of human population growth and inreasing urbanization ensures that industrial fertilizer production will continue at high and likely accelerating rate for decades in order to meet the escalating demand for food. This is the present case in the Amazon Basin Rainforest Ecosystem (ABRE). Due to migration and other factors, the indigenous population in the ABRE is currently rising. In addition, improved transportation is attracting more migrants into the area. Their destruction of rainforest land and their use of fertilizers further impacts the nitrogen cycle.
Fossil Fuel Burning:The burning of fossil fuels such as coal and oil relases previously fixed nitrogen back to the atmosphere in the form of nitrogen- based trace gases such as nitric oxide. High- temperature combustion also fixes a small amount of atmospheric nitrogen direcly. Together, th eoperation of automobiles, factories, power plants, and other combustion processes emit more than 20 Tg per yar of fixed nitrogen to the atmosphere. (9)
Mobilization of Stored Nitrogen: Besides enhancing fixation and releasing nitrogen from geological reservoirs, human activities,such as burning of forests, wood fuels, and grasslands also liberate nitrogen from long- term biological sotrage pools sucha as soil organic matter and tree trunks, contributing further to the release of biologically available nitrogen.
One of the major consequence of human- driven alterations in the nitrogen cycle has been regional and global changes in the chemistry of the atmosphere- specifically increased emissions of nitrogen- based tace gases such as nitrous oxide, nitric oxide, and ammonia. (10) If left unattended, these fases can have detrimental long term effects. Nitrous oxide contributes to the greenhouse affect while nitric oxide is an important precursor of acid rain and photochemical smog.
Nitrous oxide is a very effective heat- trapping gas in the atmosphere. This is in part becuse it absorbs outgoing radiant heat from the Earth in infrared wavelengths that are not captured by other major greenhouse gases. Although it is fairly unreactive in the lower atmosphere, when it rises into the stratosphere, it can trigger chain reactions that deplete and thin the stratoshperic ozone layer that shields the Earth from damagin ultraviolet radiation. (11)
Both nitric oxide and ammonia are highly reactive in the lower atmosphere. Nitric oxide plays several crucial roles in atmospheric chemistry, including catalyzing the formation of photochemical smog. In the presence of sunlight, nitric oxide and oxygen react with hydrocarbons emitted by automobile exhausts to form ozone- the most dangerous component of smog. (12) Ground- level ozone has serious detrimental effects on human health as well as the health and productivity of crops and forests.
Nitric oxides, along with other nonmetal oxides, can be transformed in the atmosphere into nitric acid and sulfuric acid (for example), which are major components of acid rain.
Among these many sources of nitric oxide emmision, combustion is the dominant one.
A chief danger of the increasing levels of nitrogen is the threat that it poses to the carbon cycle. Experiments in Europe and America indicate that a large portion of the extra nitrogen retained by forest, wetland, and tundra ecosystems stimulates carbon uptake and storage. On the other hand, this nitrogen can also stimulate microbial decomposition and thus releases of carbon from soil organic matter. On balance, however, te carbon uptake through new plant growth appears to exceed the carbon losses, especcially in forests. The most recent analysis of the global carbon cycle by the Integovernmental Panel on Climate Change concluded that nitrogen depostion could represent a major component of the missing carbon sink. (13)
Nitrogen Saturation: There is a limit to how much plant growth can be increased by nitrogen fertilization. Eventually, when the natural nitrogen dificiencies in an ecosystem are fully relived, plant growth becomes limited by sparsit of other nutrients, such as phosporus, calcium, and water. When the vegetation can no longer respond to gurther additions of nitrogen, the ecosystem reaches a state described as nitrogen saturation. When an ecosystem is fully nitrogen- saturated and its soils, plants, and microbes cannot use or retain any more, all new nitrogen deposits will be dispersed to streams, groundwater, and the atmosphere. (14)
Nitrogen saturation has a number of damaging consequenses for the health and functioning of ecosystems. These effects were first observed in Europe when scientists noticced an large increacce in nitrate concentraton in some lakes an streams.
As ammonium builds up in the soil, it is increasingly converted to nitrate by bacterial action. This process releases hydrogen ions and helps acidify the soil. The biuldup of nitrate enhangces emissions of nitrous oxides from the soil and also encourages leaching of highly water- soluble nitrate into streams or groundwater. As thse negatively charged nitrates weep away, they carry with them positively charged alkaline minerals such as calcium, magnesium, and potassium. This in turn alters the soil composition and depletes it of other nurtrients necessary for healthy plant growth. As calcium is depleted nd teh soil acidified, aluminum ions are mobilized, eventually reaching toxic oncentrations that can damage tree roots or kill fish if the aluminum wahses into streams. Trees growing in soils replete with nitrogen. However, starved of calcium, magnesium, and potasium, a nutrient imbalance arises in te roots an dleaves. This can potetially reduce the photosynthetic rate and efficiency of the plant, stunt its growth, and even increase tree death. (15)
Nitrogen saturation plays a deeper role and also influences
biodiversity, species mix, and aquatic ecosystems. For more information on
this, go here
Carbon is stored in the following five major sinks:
(1) As organic molecules found in the biosphere
(2) As the gas carbon dioxide in the atmosphere
(3) As organic matter in soils
(4) In the Lithosphere as fossil fuels and sedimentary rock
(5) In the oceans as dissolved atmospheric carbon dioxide and as calcium carbonate shells in marine organisms. (16)
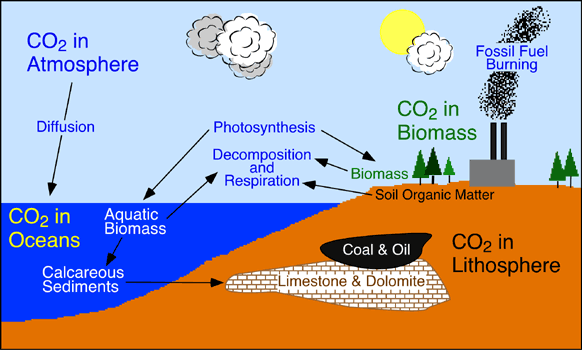
The carbon cycle is the circulation of carbon in the form of the simple element and its compounds through nature. The source of carbon in living things is carbon dioxide (CO2), from air or dissolved in water. Algae and green plants (producers) use CO2 in photosynthesis to make carbohydrates, used in the processes of metabolism tomake all other compounds in their tissues and those of animals that consume them (herbivores). The carbon may pass through several levels of herbivores and carnivores (consumers). Animals and (at night) plants return the CO2 to the atmosphere as a byproduct of respiration. The carbon in animal wastes and the bodies of organisms is released as CO2, in a series of steps, by decay organisms (decomposers), chiefly bacteria and fungi. (17) Some organic carbon (the remains of organisms) has accumulated in the earth's crust in fossil fuels, limestone, and coral. The carbon of fossil fuels, removed from the cycle in prehistoric times, is being returned in vast quantities as CO2 via industrial and agricultural processes, some accumulating in the oceans as dissolved carbonates and some staying in the atmosphere Here is an illustration of the carbon cycle (18)
The amount of carbon dioxide in teh atmosphere has been steadily increasing. The graph below whos the carbon dioxide conccentrations at the summit of Mauna Loa in Hawaii from 1958 through 1999. (19)
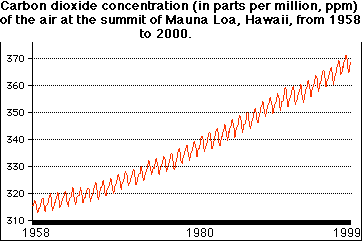
The carbon dioxide content change in the glacial ice of Greenland suggests that this increase in carbon dioxide probably began with the start of the industrial revolution. Possible human causes include: The burning of fossil fuels and the clearing and buring of forests, (especially in the tropics).These effects play a sifnificant role in global warming. Carbon dioxide is a good absorber of heat. Therefore, carbon dioxide is transparent tends to retard the radiation of heat from teh earth back into space. This phenomenon is known as the greehouse effect. A simplified diagram of the greenhouse affect is shown below.

Basically, the greenhouse effect is the result of heat absorption by ccertain gases in the atmospherek and the downward re- radiation of some of this heat. The most abundant greenhouse gas is water vapor, followed my carbon dioxide and other trace gases. An increase in the concentration of greenhouse gases, mainly carbon dioxide, has led to global increases in temperatures.
Rainforests absorb so much of the earth's carbon dioxide that scientists refer to them as carbon sinks. The largest carbon sink in the world today is the ABRE. However, deforestation is altering the carbon dioxide absorbing capabilities of the ABRE. Deforestation releases carbon dioxide into the air; it also reduces the amount of trees originally present. These two effects combined result in deminishing carbon sinks which in turn lead to and even more increase in global carbon dioxide concentrations.
These increasing temperatures have a wide array of affects on the rainforest. An article in Nature magazin predicts a dramatic collapse of the Amazonian Rainforest as a consequence of climate chance. Assuming that fossil fuel emissions increase as they are now, the process will begin within a few decades. (20) The cause will be the combined effect of reduced precipitation and increased respiration.
Before I begin my discussion on humidity, I will give a brief overview of the water cycle, as it relates to my explanation of humidity.
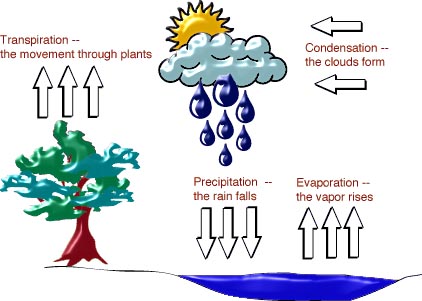
The water cycle can be separated into six main processes: evaporation, condensation, precipitation, surface runoff, infiltration, and transpiration.(21)
EVAPORATION: Evaporation is the process in which a liquid is changed into a gas. It is important to realize that this is a physical change and not a chemical change. What this basically means is that the chemical composition of the liquid is NOT changed during this process. Evaporation is an endothermic process. This means that energy must be added for evaporation to occur. Calculation of Gibb's Free energy for this process reveals that evaporation is not a spontanious process at twenty five degrees celcius and one atmosphere of pressure.
Now we must consider what affects the boiling point (the boiling point is the temperature and pressure at wich evaporation occurs). All gases condense (condensation is the reverse of evaporation) to a liquid under high pressure. This implies that evaporation is more favorable at lower pressures. From our everyday experience, we know that evaporation and condensation depends on temperature as well. Now using the equation for Gibb's free energy, we can deduce that temperature has a greater affect on evaporation than pressure.
CONDENSATION: As previously mentioned, condensation is the reverse of evaporation; it is the process by which water is changed into a gas. This too is a physical change. At a constant pressure, condensation is favored at cooler temperatures.
PRECIPITATION: When the temperature and atmospheric conditions attain a certain value, small droples of water in clouds from larger droptlets and precipitation occurs.(22)
SURFACE RUNOFF: "Much of the water taht returns to Earth as precipitation runs off the surface of the land, and flows downhill into streams, rivers, ponds, and lakes."(23) Small streams join rives, which in turn flow into the oceans. (24)
INFILTRATION: Infiltration is a critical process. During infiltration, rain water soaks into the ground, goes through the soil and underlying rock layers. Some of this water eventually returns to the surface, and some of it remains underground. The water that remain underground is called groundwater.(25)
TRANSPIRATION: This last process is very important to the water cycle. Plants absorb water through their roots and eventually realease it (they transpire) back into the atmosphere in the form of water vapor. to a lesser extent, transpiration also occurs in mamals through perspiration. Sweat released uses the heat from the body as an energy source and becomes water vapor.
Humidity refers to the amount of water vapor present in the air. It can be described in many ways, including relative humidity. Relative humidity is the amount of water vapor in the air compared to the amount of water vapor needed to make the air saturated at the current air temperature. (26) Recall that saturation implies equilibrium. That is, the maximum amount that can be dissolved or included in the mixture is present. For example. When the maximum amout of sodium chloride (table salt) that can be dissolve in water is present (in the solution), the solution is said to be saturated. Humidy levels can be calculated in several ways. To view a list of equations used to calculate humidity, click here.
It is important to note that warmer air can hold more water vapor than colder air. This is consistent with general equilibrium trends- solubility, and thus the amount of solute needed to reach saturation, tends increases with increasing temperature. This means that a change in atmospheric temperature in the ABRE will affect the humidity levels of the ABRE. Because plants and animals (consider amphibians as anexample)rely heavily on humidity for certain physiological functions, it can be concluded that the net effect will include changes in plant and animal health.
The most influential condition in predicting the distinct mixture of organisms that can survive within the ABRE is the climate. (27) Specifically, the amount of precipitation limits the growth of primary producers which in turn support the rest of the ABRE. Although the amount of rainfall is not the same at each specific region in the ABRE, there still exists a minimun amout of moisture needed to sustain life in this ecosystem. This level is generally acceppted as 200cm of rain per year. (28) Temperatures in the forests are around 26 degrees celcius (78.8 degrees farenheit or 299.15 Kelvins). (29) Under these conditions, the humidity level ranges 65 to 96 percent. (30)As mentioned earlier, the temperature and humidity levels affect both the plant vegetation and animal biodiversity of the area.
One of the biggest threats to the humidity of the ABRE is deforestation. Essentially, deforestation destroys the canopy. Because the canopy is no longer there to protect the understory and floor from the sun's radiation, the soil there dries out. In addition, the temperature there also increases. This can have many long term affects. Consider for example a cool breeze coming from the ocean. Once it reaches the area where the canopy was destroyed, it pushes up the warm air. This is a commmon source of tornadoes, however, it is important to note that this condition does not guarantee the formation of tornadoes in the amazon- other factors, which also lead to the formation of tornadoes are not present in the ABRE. Rather, this leads to less cloud formation. Several different models and studies indicate that deforestation will lead to a reduction in average rainfrall and increaed surface temperature (31). This is mainly due to decreases in humidity levels. As Philip Ball mentions in his article "Making Waves", trees are responsible for returning huge amounts of water into the air as water vapor, which then condense to form cloud and rain. With out trees, far less water vapor reaches teh atmosphere, and as a result, there is less rainfall over the ABRE(32). This prediction is proven by a study performed by Willian E. Dietrich, Donald M. Windor, and Thomas Dunne.
Barro Colorado Island in Panama is a hill protruding 137m above Gatun Lake. The climate of this tropical forest is very similar to the climate of the ABRE. The average annual temperature is twenty- seven degrees celcius, and th eyearly rainfall averages aroun 260 centimeters. Barro Colorado also shares many characteristics of the land of the ABRE. Because of these similarity, Barro Colorado Island is a good model for the ABRE. Using mathematical formuls, the average daily solar radiation at teh earth's surface was calculated. What was discovered was that as this value increased, the relative humidity decreased, the average daily temperatue increased, and the evaporation due to mass tranfer decreased (these trend was general). These trends confirms that there exists a great probabily that the predictions made earlier are accurate.
The level of these gases (Nitrogen gas, Carbon Dioxide, etc) can be monitored with the satellite Envisat. This is a satellite devoted to environmental studies, primarily in the field of atmospheric chemistry. By monitoring theses gases, one can assess the affects that increases or decreases in their concentrations will have on the cycle. For more information on this satellite, visit rain Micheal's (Mike) webpage. You can do this by clicking on "group members" on the home page.
WORKS CITED
(1) Pidwirny, Micheal J. Fundamentals of Physical Geography. Copyright 1996-2002.
(2) Ibid
(3) Ibid
(4) Kimall, John W. Biology: The Nitrogen Cycle. Wm C. Brown, Washington DC: 1994.
http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/N/Nitrogencycle.html
(5) Ibid
(6) Ibid
(7) Ibid
(8) Peter M. Vitousek, John Aber, Robert W. Howarth, Gene E. Likens, Pamela A. Matson, David W. Schindler, Willian H. Schlesinger, and G. David Tilman. Ecological Applications: Human Alteration of the Global Nitrogen Cycle: Causes and Consequences. Volume 7 August, 1997.
(9) Ibid
10) Ibid
(11) Ibid
(12) Ibid
(13) Ibid
(14) Ibid
(15) Ibid
(16) Pidwirny, Micheal J. Fundamentals of Physical Geography
(17) Ibid
(18) Ibid
(19) http://www.science.uwaterloo.ca/earth/waton/mauna.html
(20)Rossiter, Al Jr. Study Predicts Amazon Deforestation could Affect Climate in US. http://www.eurekalert.org/pub_releases/2002-10/du-spa102402.php
(21) The Water Cycle. Copyright 1995-1998 by The Evergreen http://mbgnet.mobot.org/fresh/cycle/index.htm
(21) Ibid
(22) Ibid
(23) Ibid
(24) Ibid
(25) Ibid
(26) USA Today. Water Basics: Understanding Humidity. http://www.usatoday.com/weather/whumdef.htm
(27) Hall, Jennifer. The Affect of Tropical Farming Practices on the Amazon Rainforest http://www.personal.psu.edu/users/j/m/jmh280/title.html
(28) Ibid
(29) Ibid
(30) Ibid
(31) Hastenrath, 1991 adn Shukla 1990
(32) Dietrich, William E., Donald M. Windsor, Thomas Dunne, A. Sanley Rand, and Willian M. Rand. The Ecology of a Tropical Forest. Geology, Climate, and Hydrology of Barro Colorado Island, Variation in Rainfall of Barro Colorado Island. Leigh, Egbert G., A. Stanley Rand and Donald M, Windsor. The Smithsonian Institte, 1996.
