THE NITROGEN CYCLE
Background: Plants require nitrogen for healthy growth. Most of the nitrogen in organic matter is in a form that plants cannot use. Bacteria found in the siol converts these organic forms of nitrogen into forms that the plant can use. When plants die, they decompose and become part of the organic matter of the soil and the cycle is repeated again.
The nitrogen cycle is one of the most important nutrient cycles found in terrestrial ecosystems. Nitrogen is the building block of many complex molecules formed by plants and animals; some examples are amino acids, proteins, and nucleic acids used in DNA.
Most of the world's nitrogen is located in the atmosphere as N2 gas. Still, atmospheric nitrogen is unuseful to the plants. This is because plants can only consume nitrogen in two solid forms: the ammonium anion (NH4+) and the nitrate anion (NO3-). However, the former is more preferred than the latter because large concentrations of ammonium is extremely toxic. (1)
In most ecosystems, nitrogen is primarily sotred in living and dead organic matter. This nitrogen is converted into inorganic forms (forms usable by plants) when it re- enters the biogeochemical cycle (Cycling of chemicals through the biosphere, lithosphere, hydrosphere, and atmosphere) via decomposition. (2) Decompositoin is achieved by various decomposers found in the upper soil layer. They alter the nitrogen found in organic matter into such forms as ammonium salts. This process, known as mineralization, is carried out by a variety of bacteria, actinomycetes, and fungi. (3)
Animals also require a certain amount of nitrogen in their system for survival. Animals, however, secure their nitrogen fixation through plants or other animals that have fed on plants.
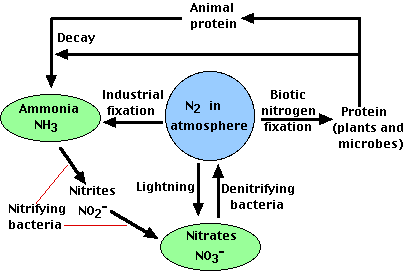
Four processes participate in the cycling of nitrogen through the biosphere: Nitrogen Fixation Decay Nitrification Denitrification (4)
Nitrogen Fixation:The nitrogen molecule (N2) is inert. It is also stable because of the strengh of the tripple bond that it possesses. Thus, breaking it apart will require a substancial amount of energy. Most industrial fixation is achieved under great pressure, at temperatures of 600 degrees celcius, and with the use of a catalyst. Under these conditions, atmospheric nitrogen and hydrogen can be combined to form ammonia. This can be used directly as a fertilizer, however, it is further processed into more useful forms such as urea and ammonium nitrate.
The ability to fix nitrogen is found only in certain bacteria. The first step in this process produces ammonia. However it is quickly incorporated into protein and other organic nitrogen compounds.
Decay:When plants die, they decay and leave behind organic forms of nitrogen. This is changed into more useful inorganic forms by certain bacteria.
Nitrification:Ammonia can be taken up directly by plants- usually through their roots. However, most of the ammonia produced by decay is convertd into nitrates. This is accomplished in two steps: *Bacteria of the genus Nitrosomonas oxidize ammonia to nitrites. *Bacteria of the genus Nitrobacter oxidize nitrites to nitrates. These two groups of bacteria are called nitrifying bacteria. (5) Through their activities, nitrogen is made available to the roots of plants.
Denitrification:The previous processes remove nitrogen from the atmosphere and pass it through the ecosystems. Denitrification reduces nitrates to nitrogen gas, thus replenishing the atmosphere. Denitrification is achieved by bacteria that live deep in the soil and in aquatic sediments. Because the conditions are anaerobic, they use the nitrates as an alternatie to oxigen for the final electron accceptor in their respiration. (5)
Another process, called volatilization turns urea fertilizers and manures on the soil surface into gases that also join the atmosphere. (5) This completes the nitrogen cycle.
The nitrogen cycle:
(image: John Kimball)
(1) Pidwirny, Micheal J. Fundamentals of Physical Geography. Copyright 1996-2002.
(2) Ibid
(3) Ibid
(4) Kimall, John W. Biology: The Nitrogen Cycle. Wm C. Brown, Washington DC: 1994.
http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/N/NitrogenCycle.html
(5) Ibid

