UNIFIED ENGINEERING Fall 2003
Ian A. Waitz
Problem T11 (Unified Thermodynamics)
A device called a heat exchanger is shown below. Assume that both the hot side flow and the cold side flow behave as ideal gases with R=287 J/kg-K, cp=1003.5 J/kg-K, and cv = 716.5 J/kg-K.
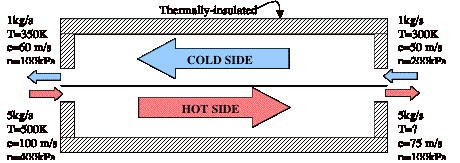
a) What is the temperature at the exit of the hot side flow? (LO# 4)
b) This is a quote from Thermodynamics for Engineers by Wong,©2000 by CRC Press. “No work is done in a heat exchanger.” Do you agree or disagree and why? Please substantiate your argument with a calculation. (LO# 4)
c) Is the process in this device reversible or irreversible and why? (Do not do a calculation; answer with a few sentences) (LO# 5)
d) Describe the energy exchange processes in the device in terms of various forms of energy, heat and work. (LO# 2)