Ian A. Waitz
Problem T3. (Unified Thermodynamics)
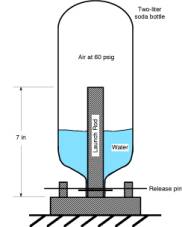
Consider the following two processes for charging a water rocket with high pressure air. For each, the initial condition is the same: 101.3 kPa and T=288 K. Assume that the bottle walls are rigid, the bottle volume is 2.3 x 10-3 m3 and that it is 40% filled with water. Assume that cp = 1003.5 J/kg-K, cv = 716.5 J/kg-K, and R = 287 J/kg-K.
Process 1: isothermal compression to a pressure of 60 psig (413.7 kPa)
Process 2: adiabatic compression to 60 psig
Process 3: adiabatic compression to 60 psig followed by constant volume cooling to T = 288 K
a) Sketch each process on the same p-v diagram.
b) How much work is done by the gas for each process?
c) How much heat is transferred to the gas for each process?
d) Which process do you think is most advantageous if your objectiv e is to maximize the height that the water rocket will go? Why? Note that during the launch, the air in the bottle can be assumed to expand via a quasi-static, adiabatic process. Support your answer with a calculation.
(LO#4, LO#5)