Immersion Heater Demo Evaluation
Christopher Gouldstone
9/17/00
A store-bought immersion-heating unit, typically used to heat single cups of water for coffee or tea, has a displayed power rating of 125 W. Such a unit was placed in a medium-sized, black mug, along with a laboratory thermometer. Once plugged in, the unit supplied heat to the water in the mug. The mass of fluid at the start of the heating process was 200g. Immediately after the test was concluded, 195g remained. The heating process continued for 10 minutes, during which time temperature measurements were taken every 30 seconds.
The model used to predict the behavior of the system made the following assumptions:
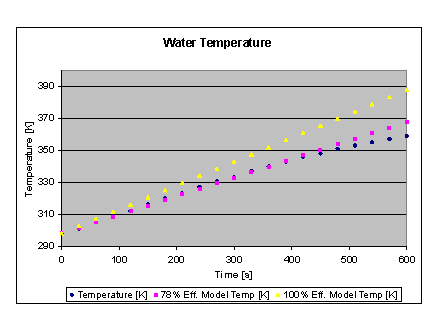
Assuming Q-dot = 125 W, the model predicts a 9 K/min, linear temperature rise. Comparison with actual data shows this to be an overestimate, as can be expected due to the nature of the assumptions made.
Fitting a line along the observed data curve, a 78% efficient heating process matches the observed data over most of the temperature range measured (~40K), so the observed data behaves more like the model for a 100W heater would.
The actual data is plotted below, along with the data predicted by the analytical models for both 100% efficient, and 78% processes.

Pitfalls: In order to obtain a marked increase in temperature (~50K) in a comparatively short time (~10 minutes), only a modest mass of water should be used. A regular mug, somewhat full, holds approximately 200g of water. The heater should be immersed at the time it is plugged in, for safety reasons. Only a modest amount of time (~30s) is necessary for the temperature change to ‘ramp-up’.
The thermometer and heater should be maintained in a constant orientation with respect to each other. Moving the thermometer back and forth from the heater can cause erratic readings, which do not necessarily reflect the water temperature. Since a relatively small mass of fluid is used in the experiment, spatial temperature variations within the fluid should be small. If observations are made sufficiently far from the heater itself, the measurements should be largely indicative of the water temperature.
Required apparatus:
One (1) 125-watt Travel Immersion Heater
One (1) Extension cord (need not be grounded)
One (1) Lab thermometer
One (1) Stopwatch
One (1) Medium-sized Mug
One (1) Scale (to measure mass change)
200g Tap Water
Support Apparatus (tape, elastic bands, etc)