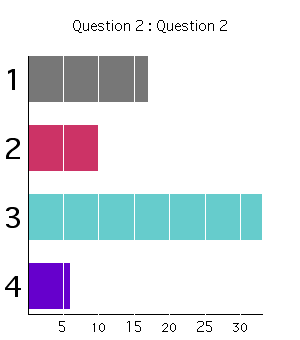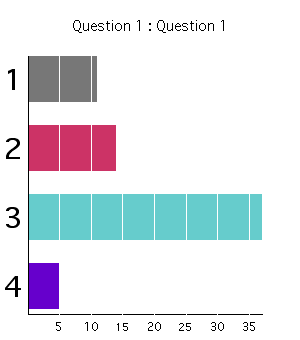
Chapter 4 Question 14 Answer:
(3) Cv < Cp
Gases are compressible. If energy is added to them at constant pressure, they expand (pv=RT). As they expand, some of the energy goes towards doing work. When a gas is constrained (constant volume), relatively less energy needs to be added to them to change their temperature (since the work is zero).
Class Response (2003):
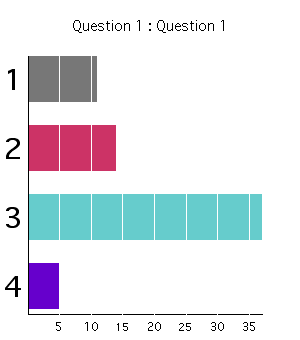
Class Response (2002):