4.1 Reversibility and Irreversibility in Natural Processes
We wish to characterize the ``direction'' of natural processes;
there is a basic ``directionality'' in nature. We start by examining
a flywheel in a fluid filled insulated enclosure as shown in
Figure 4.1.
Figure 4.1:
Flywheel in insulated enclosure at
initial and final states
|
|
A question to be asked is whether we could start with state B and
then let events proceed to state A? Why or why not? The first law
does not prohibit this.
The characteristics of state A are that the energy is in an
organized form, the molecules in the flywheel have some circular
motion, and we could extract some work by using the flywheel kinetic
energy to lift a weight. In state B, in contrast, the energy is
associated with disorganized motion on a molecular scale. The
temperature of the fluid and flywheel are higher than in state A, so
we could probably get some work out by using a Carnot cycle, but it
would be much less than the work we could extract in state A. There
is a qualitative difference between these states, which we need to
be able to describe more precisely.
Muddy Points
Why is the ability to do work decreased in B? How do we know?
(MP 4.1)
Another example is a system composed of many bricks, half at a high
temperature 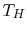 and half at a low temperature
and half at a low temperature 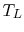 , as shown in
Figure 4.2. With the bricks separated thermally, we
have the ability to obtain work by running a cycle between the two
temperatures. Suppose we put two bricks together. Using the first
law we can write
, as shown in
Figure 4.2. With the bricks separated thermally, we
have the ability to obtain work by running a cycle between the two
temperatures. Suppose we put two bricks together. Using the first
law we can write
where 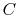 is the ``heat capacity''
is the ``heat capacity''
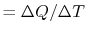 . (For
solids the heat capacities (specific heats) at constant pressure and
constant volume are essentially the same.) We have lost the ability
to get work out of these two bricks.
. (For
solids the heat capacities (specific heats) at constant pressure and
constant volume are essentially the same.) We have lost the ability
to get work out of these two bricks.
Figure 4.2:
Bricks separated by a temperature
difference
|
|
Can we restore the system to the original state without contact with
the outside? The answer is no. Can we restore the system to the
original state with contact with the outside? The answer is yes. We
could run a refrigerator to take heat out of one brick and put it
into the other, but we would have to do work.
We can think of the overall process involving the system (the two
bricks in an insulated setting) and the surroundings (the rest of
the universe) as:
- System is changed,
- Surroundings are unchanged.
The composite system (system and the surroundings) is changed by
putting the bricks together. The process is not reversible --
there is no way to undo the change and leave no mark on the
surroundings.
What is the measure of change in the surroundings?
- Energy? This is conserved.
- Ability to do work? This is decreased.
The measurement and characterization of this type of change - of
losing the ability to do work - is the subject of the second law of
thermodynamics. [VW, S & B: 6.3-6.4]
UnifiedTP
| 
