A C++ Chemical Equilibrium Program
In the program below, the equilibrate function is called to set the gas to a state of chemical equilibrium, holding the temperature and pressure fixed.
This function is declared in the equilibrium.h header file.
#include <cantera/Cantera.h> #include <cantera/equilibrium.h> void equil_demo() { ThermoPhase* gas = newPhase("h2o2.cti","ohmech"); gas->setState_TPX(1500.0, 2.0*OneAtm, "O2:1.0, H2:3.0, AR:1.0"); equilibrate(*gas, "TP"); cout << report(*gas) << endl; } int main() { try { equil_demo(); } catch (CanteraError) { showErrors(); } }
The program output is:
temperature 1500 K
pressure 202650 Pa
density 0.316828 kg/m^3
mean mol. weight 19.4985 amu
1 kg 1 kmol
----------- ------------
enthalpy -4.17903e+06 -8.149e+07 J
internal energy -4.81866e+06 -9.396e+07 J
entropy 11283.3 2.2e+05 J/K
Gibbs function -2.1104e+07 -4.115e+08 J
heat capacity c_p 1893.06 3.691e+04 J/K
heat capacity c_v 1466.65 2.86e+04 J/K
X Y Chem. Pot. / RT
------------- ------------ ------------
H2 0.249996 0.0258462 -19.2954
H 6.22521e-06 3.218e-07 -9.64768
O 7.66933e-12 6.29302e-12 -26.3767
O2 7.1586e-12 1.17479e-11 -52.7533
OH 3.55353e-07 3.09952e-07 -36.0243
H2O 0.499998 0.461963 -45.672
HO2 7.30338e-15 1.2363e-14 -62.401
H2O2 3.95781e-13 6.90429e-13 -72.0487
AR 0.249999 0.51219 -21.3391
How can we tell that this is really a state of chemical equilibrium? Well, by applying the equation of reaction equilibrium to formation reactions from the elements, it is straightforward to show that
![\[ \mu_k = \sum_m \lambda_m a_{km}. \]](form_531.png)
where 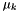 is the chemical potential of species k,
is the chemical potential of species k, 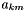 is the number of atoms of element m in species k, and
is the number of atoms of element m in species k, and 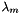 is the chemical potential of the elemental species per atom (the so-called "element potential"). In other words, the chemical potential of each species in an equilibrium state is a linear sum of contributions from each atom. We see that this is true in the output above -- the chemical potential of H2 is exactly twice that of H, the chemical potential for OH is the sum of the values for H and O, the value for H2O2 is twice as large as the value for OH, and so on.
is the chemical potential of the elemental species per atom (the so-called "element potential"). In other words, the chemical potential of each species in an equilibrium state is a linear sum of contributions from each atom. We see that this is true in the output above -- the chemical potential of H2 is exactly twice that of H, the chemical potential for OH is the sum of the values for H and O, the value for H2O2 is twice as large as the value for OH, and so on.
We'll see later how the equilibrate function really works. For now, though, the important points are these:
- The
equilibrateprocedure operates on an object, setting its state to a chemical equilibrium state. - To use
equilibrate, you need to include the equilibrium.h header file.
Return to Getting Started with Cantera in C++
 1.6.3
1.6.3