
Liquid water and steam (gaseous water) are two phases of the same substance. To boil water is to cause a phase transition because we start with one phase (liquid) and end up with another. There’s another special thing about this phase transition – there is both liquid and gaseous water in the pot at the same time (and again, we can clearly distinguish liquid water from gaseous steam). If we went in the opposite direction – cooling the steam to liquid – we’d see droplets of water condense bit by bit, just like we see bubbles of steam appear. Either way, the transition doesn't happen everywhere all at once. It happens in bits and pieces, and takes some time and a certain amount of energy (in the form of heat) before all the water has turned to gas.
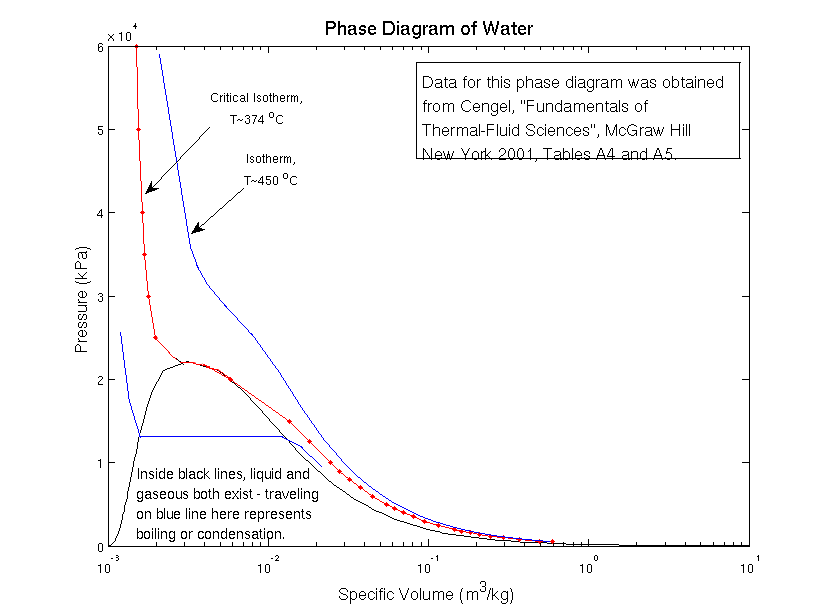
Look at the phase diagram above; it shows you at what temperatures and pressures water exists as a solid (ice), as a liquid, and as a gas (steam). A red line has been drawn to show the boiling transition on the phase diagram.

Ask yourself:
Click here to hide the answers.
