Critical Point
 Now here’s the weird part – at high temperatures and pressures, it becomes harder to tell liquid water and gaseous water apart. There’s a special mix of temperature and pressure – we call it the critical point – where the difference between liquid and gas ceases to exist. For water, this happens at 374 °C (705 °F) and 218 atmospheres (normal air pressure is one atmosphere at sea level!). For carbon dioxide, it’s 31.1 °C (-1.6 °F) and 73 atmospheres. For nitrogen, -147 °C (-322 °F) and 33 atmospheres. No wonder it seems so funky to us! For a bunch of the substances in our world, the critical point happens at conditions extreme for human beings. We’d never see it in the kitchen!
Now here’s the weird part – at high temperatures and pressures, it becomes harder to tell liquid water and gaseous water apart. There’s a special mix of temperature and pressure – we call it the critical point – where the difference between liquid and gas ceases to exist. For water, this happens at 374 °C (705 °F) and 218 atmospheres (normal air pressure is one atmosphere at sea level!). For carbon dioxide, it’s 31.1 °C (-1.6 °F) and 73 atmospheres. For nitrogen, -147 °C (-322 °F) and 33 atmospheres. No wonder it seems so funky to us! For a bunch of the substances in our world, the critical point happens at conditions extreme for human beings. We’d never see it in the kitchen!
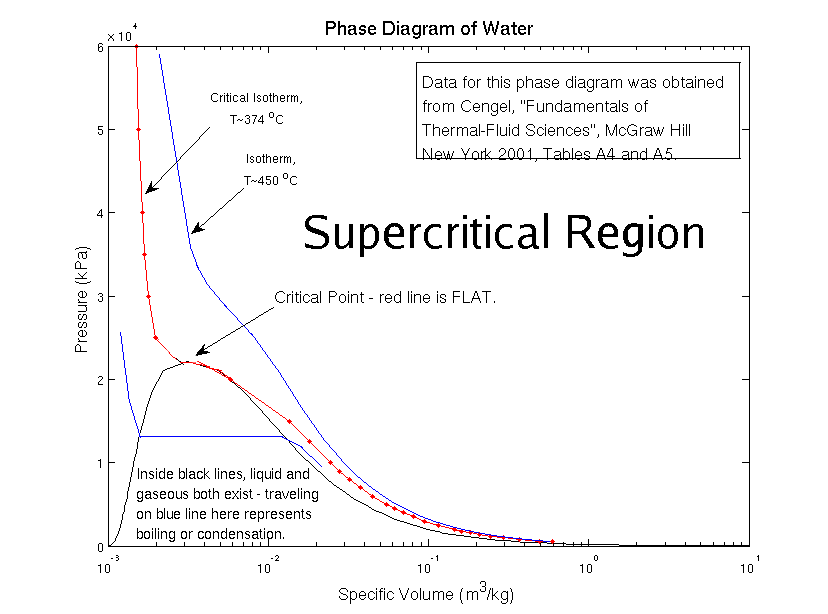 In the phase diagram, the area where liquid and gas combine into the same thing is labeled as the “supercritical” region – in this case, “super” means “above”.
In the phase diagram, the area where liquid and gas combine into the same thing is labeled as the “supercritical” region – in this case, “super” means “above”.

Quizlet:
What was the important difference between liquid and gas in the first place?
(a) One is wet and the other dry.
(b) One is cold and the other hot.
(c) One is more dense than the other.
(d) There's no difference!
Click here for the answer!
Order Parameter
The word, density, keeps coming up. Remember what it means? It's how much matter (stuff) there is in a certain volume (space). For this liquid-gas transition, density plays the role of the order parameter - basically, some measurement which is different in the two phases and so can be used to tell them apart. Different situations call for different order parameters; for example, a good order parameter for a magnet is the magnetization - how much the magnetic (dipole) fields from all of the magnetic system elements ("spins" instead of molecules) tend to align. For a binary fluid mixture like that described in a few pages, the order parameter is more complicated - it's a combination of the total density of the combined fluid with the relative densities for each fluid ("relative" density meaning that we only count the amount of one type of fluid in a given unit of volume).
At the critical point, the density of liquid and gas become the same. And so the difference between these two phases disappears – they become a single, supercritical phase.
 Now here’s the weird part – at high temperatures and pressures, it becomes harder to tell liquid water and gaseous water apart. There’s a special mix of temperature and pressure – we call it the critical point – where the difference between liquid and gas ceases to exist. For water, this happens at 374 °C (705 °F) and 218 atmospheres (normal air pressure is one atmosphere at sea level!). For carbon dioxide, it’s 31.1 °C (-1.6 °F) and 73 atmospheres. For nitrogen, -147 °C (-322 °F) and 33 atmospheres. No wonder it seems so funky to us! For a bunch of the substances in our world, the critical point happens at conditions extreme for human beings. We’d never see it in the kitchen!
Now here’s the weird part – at high temperatures and pressures, it becomes harder to tell liquid water and gaseous water apart. There’s a special mix of temperature and pressure – we call it the critical point – where the difference between liquid and gas ceases to exist. For water, this happens at 374 °C (705 °F) and 218 atmospheres (normal air pressure is one atmosphere at sea level!). For carbon dioxide, it’s 31.1 °C (-1.6 °F) and 73 atmospheres. For nitrogen, -147 °C (-322 °F) and 33 atmospheres. No wonder it seems so funky to us! For a bunch of the substances in our world, the critical point happens at conditions extreme for human beings. We’d never see it in the kitchen!

