Clues
 Critical opalescence is a terrific clue to what’s happening at the critical point because it tells us that something is scattering visible light. Light goes into the substance, but instead of going through like normal, it bounces off into all different directions. If a normally clear substance is scattering light, something must be going on inside the substance, but what?
Critical opalescence is a terrific clue to what’s happening at the critical point because it tells us that something is scattering visible light. Light goes into the substance, but instead of going through like normal, it bounces off into all different directions. If a normally clear substance is scattering light, something must be going on inside the substance, but what?
To figure out what, we need to understand why light scatters in the first place. Important discoveries about the scattering of light were made by John Tyndall in the 1860s, and by John Strutt (Lord Rayleigh), in 1871. The thing we need to know about scattering right now is that small changes or imperfections in an otherwise uniform, transparent medium scatter light. Here, we know the medium – be it water or carbon dioxide or whatever system we’re looking at – is usually uniform, but must develop lots and lots of changes inside close to the critical point.
REMEMBER...
Remember why we were able to see the bubbles of steam in boiling liquid water? Keep this in mind – it'll come in handy....
Another important clue comes from the phase diagram.
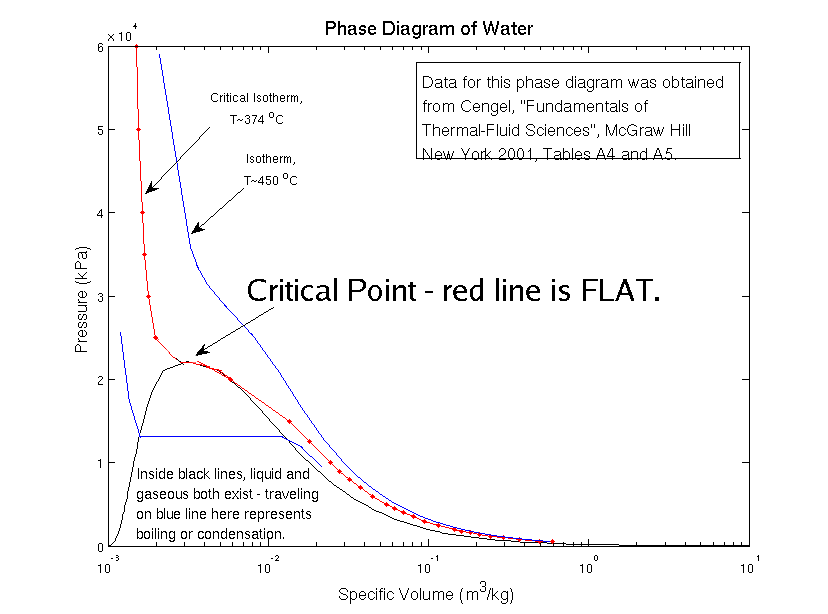
Look at the phase diagram. It is drawn with pressure on the vertical axis and volume on the horizontal axis. The lines you see show what pressure you get for a given volume at a certain temperature. They are called isotherms because each line tells you all of the pressures and volumes you get at a certain temperature (from the Greek prefix, iso, meaning same, and word, thermos, from heat).
The critical isotherm is just the isotherm drawn at the critical temperature. This isotherm is special because there is a certain temperature and pressure for which it becomes flat. Here, “flatness” is measured by the slope (rise over run) – the flatter the line is, the smaller the slope. None of the isotherms with temperature greater than critical are flat.
The inverse of the slope (one divided by the slope) – run over rise – tells you how far you move along the horizontal axis if you change your spot on the vertical axis. But remember, these axes have meanings – the horizontal axis is volume and the vertical is pressure. So the inverse of the slope tells you the change in volume for a little change in pressure. This ratio has a name – the isothermal compressibility (or how “press-able” something is at a certain temperature). Something with a small compressibility requires a lot of pressure to change its size – it is very stiff. Something with a large compressibility changes size very readily – it is very soft.
If the isotherm is very flat, and the slope very small, then the inverse of the slope is very big. In fact, on the critical isotherm, the compressibility becomes infinitely large (at least, that’s what this simple model tells us). That means that even a tiny change in pressure results in a very large change in volume. If the volume changes, but the mass stays the same, then the density changes.
The key to understanding critical opalescence is to recognize that there are always small changes, or fluctuations, in quantities like pressure or temperature. This is because the liquid or gas that we’re studying is actually composed of lots and lots and lots (and lots) of little, tiny elements.
 Critical opalescence is a terrific clue to what’s happening at the critical point because it tells us that something is scattering visible light. Light goes into the substance, but instead of going through like normal, it bounces off into all different directions. If a normally clear substance is scattering light, something must be going on inside the substance, but what?
Critical opalescence is a terrific clue to what’s happening at the critical point because it tells us that something is scattering visible light. Light goes into the substance, but instead of going through like normal, it bounces off into all different directions. If a normally clear substance is scattering light, something must be going on inside the substance, but what?