Matter

Since phase transitions occur in real physical systems, that is, in things made of matter, we should briefly discuss the nature of matter and how it is related to its constituent parts.
An atom, composed of protons, neutrons, and electrons in specific ratios, is the fundamental unit of the matter we encounter on a daily basis. Ultimately, the cause of a phase transition is to do with the fact that a system is composed an incredible number of identical constituent parts. Thus, while the atom may be the fundamental unit of matter, in understanding why a system undergoes a phase transition, its constituent parts may be atoms, or molecules, or round balls, or any other unit that can appear identically an enormous number of times. While all these things are certainly made of atoms themselves, that fact does not contribute to the onset of a phase transition. In examining the phase transition of diblock copolymers, for example, the constituent part of the system is a chain of two varieties of monomer. Thus, while each constituent part is in and of itself composed of perhaps millions of atoms, this fact is irrelevant in understanding the problem from a theoretical viewpoint. One of the fundamental notions behind the physics of interacting systems is that of course-graining, of looking at a system at an increasingly large scale to see how interactions behave. In a polymer melt the interactions between atoms are relatively unimportant compared with the interactions between chains.
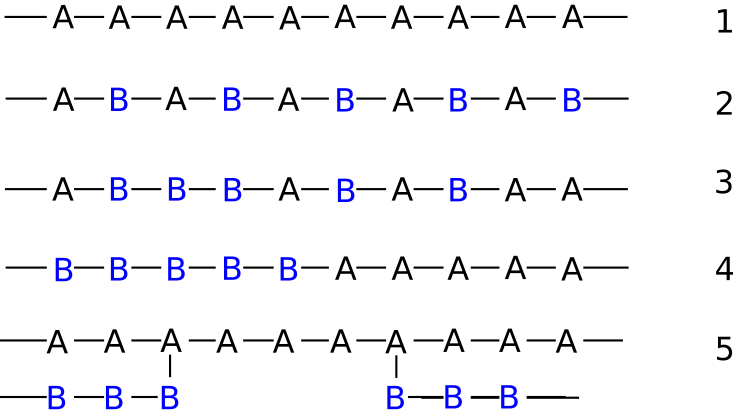
This image shows a variety of polymers composed of two monomer types A and B in several different configurations. The last two are of particular interest and are known is diblock copolymers and grafted copolymers.
While it is well understood that matter is composed of an enormous number of individual atoms, what is perhaps not appreciated is how matter obtains properties so radically different than its constituent elements. For example, consider a sheet of ordinary aluminum. Aluminum has the capacity to melt, tear, bend, and crumple. That can hardly be said for the individual atoms of which it is made. That the material obtains properties indescribable by the study of its individual components is an example of emergence, the idea that the whole is more than the sum of its parts. When atoms interact strongly with one another they have the ability to form different phases of matter. In short, a phase of matter is a collection of interacting constituents that possesses macroscopic properties, properties that are not available by the study of the constituent. So when there is a large collection of constituents, how is it they choose a particular phase to form? Again from common experience, matter composed entirely of one constituent, like water, can exist in multiple phases. Understanding this will require an understanding of the competition between energy and entropy, between order and disorder in a large system.

