Examples of Phase Transitions
A phase transition is a very general class of phenomenon. Not only are there numerous, varied, types of phase transitions, but the term has obtained mathematical significance such that 'phase transitions' can occur in systems that have no physical analogue, or are not related to the organization of matter. For example percolation is the name given to the phenomenon of liquid passing through a porous material. Below a certain critical porosity, the material would be unable to allow the liquid to pass. This phenomenon can be accurately understood using the language of phase transitions, and is thus considered as one. This generality has only made the understanding of phase transitions more important because it confirms their ubiquity.
This picture illustrates the nature of percolation. By assuming that any two dots are connected with a particular probability, there will be some critical probability above which chains of connected dots of arbitrary length will be found. These long chains are like paths allowing fluid to pass through the material.
Additionally, phase transitions can occur in exotic forms of matter that are very similar to the solid/liquid/gas transitions we know, but do not occur as obviously. For example, above a certain temperature called the Curie temperature, a magnet ceases to remain magnetic. This is a classic and important example of a phase transition, and even if it seems nothing like the boiling of water it displays all the same properties. The important qualities that it shares with other phase transitions are subtle, but of principal importance in their understanding and classification. Perhaps the most apparent property is the appearance of order in an otherwise disordered system. The following is a list of phase transitions, and the nature of the change in order
- The boiling of a liquid to a gas exhibits a decrease in order as the molecules are no longer bound and are only weakly interacting
- The freezing of a liquid to a solid exhibits an increase in order as the atoms occupy locations on a regular crystal lattice
- The appearance of a permanent magnetic moment in a ferromagnet is an example of an increase in order as the individual magnetic spins point together in the same direction
- The segregation of block copolymers in a polymer melt demonstrates an increase in order as the individual monomer chains separate and aggregate
- The alignment of liquid crystals shows an increase in directional order (although not necessarily spatial order) as the long liquid crystal molecules align and point in the same direction
- The formation of a Bose Einstein Condensate demonstrates an increase in order as the wavefunctions of the individual atoms become coherent
- Superconductivity exhibits an increase in order as pairs of electrons (Cooper pairs) act coherently
From this short list of examples, it should be clear that phase transitions occur in a wide range of materials. Physicists study phase transitions theoretically by predicting when certain classes of matter will undergo a transition from a disordered to an ordered state. One way they do this is positing the existence of a novel form of matter and classifying it based on its most general properties, like the symmetries under which it is invariant. Then, the tools of statistical physics can be used to predict the occurrence of phase transitions. Experimentalists study phase transitions by obtaining or creating the materials predicted to have phase transitions, and then placing those materials in conditions where they should transition from one phase to another. The experimentalists then look to see if the material displays the familiar (or novel) behavior of the predicted phase transitions. Additionally, the discovery of new phases of matter often brings with it important technological innovations. Liquid crystal displays are commonplace, and Bose-Einstein condensates are being studied for the purpose of locating deep reservoirs of oil. Without a deep understanding of the physics underlying phase transitions associated, it is likely the associated technology never would have developed. Physicists have predicted new phases of matter long before they were observed in experiments. Bose-Einstein condensates were predicted in 1924 by Satyendra Bose and Albert Einstein, long before they were seen experimentally.
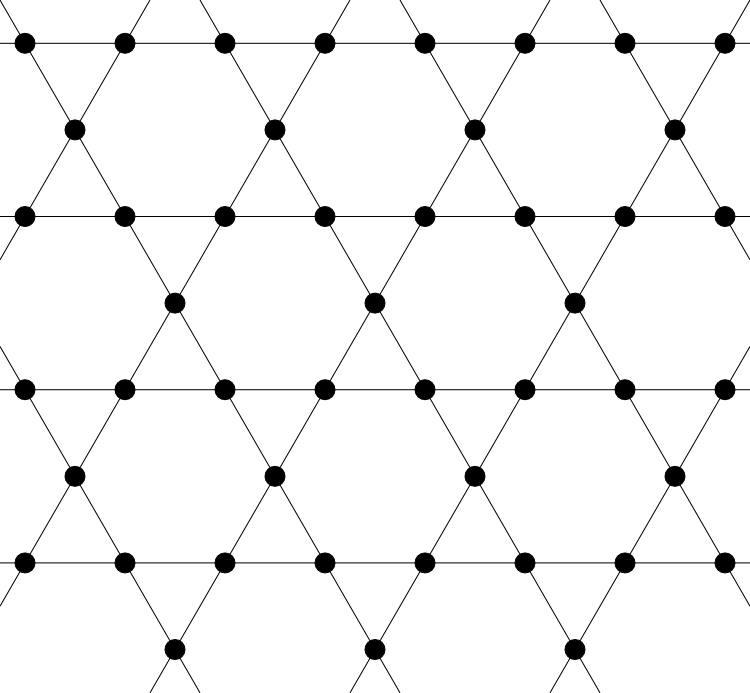
Herbertsmithite is a mineral whose constituent molecules sit on the vertices of a kagome lattice (much like the constituent atoms in salt sit on the vertices of a euclidean grid). It was predicted that such a material should exhibit a totally novel phase transition and enter a phase of matter known as a spin liquid. Experiments are currently searching for evidence to verify this.

