
Stephen Raso & Jesse Barnes
(in collaboration with Amgen Pharmaceuticals, Inc.)
The aggregation/polymerization of overexpressed proteins to form an insoluble inclusion body state is a major problem in biotechnology. Similar processes have been implicated as the causative action of many diseases, such as Alzheimer's disease, sickle-cell anemia, and emphysema. The mechanism of aggregation and conformation(s) of polypeptide chains in the aggregated state are not yet well defined for any protein.
Granulocyte-colony stimulating factor (G-CSF) is a protein hormone commonly administered to cancer patients to combat neutropenia (low white blood cell count) during chemotherapy. The protein's propensity to aggregate rapidly under physiological conditions (pH 7.0, 37 °C) can complicate its therapeutic use. Therefore, we are trying to elucidate the specific mechanism of G-CSF aggregation as well as learn more about the polypeptide conformation in the aggregated state.
The rate of G-CSF aggregation is dependent on the protein concentration (Fig. 1), and intermolecular disulfide bonds form within the aggregated state. Substitution of Cys17 (the only free thiol in the protein) eliminates the formation of covalently linked aggregates and slows the overall rate of aggregation. Thus suggesting that the cyteine at postion 17 plays in active role in the aggregation mechanism.

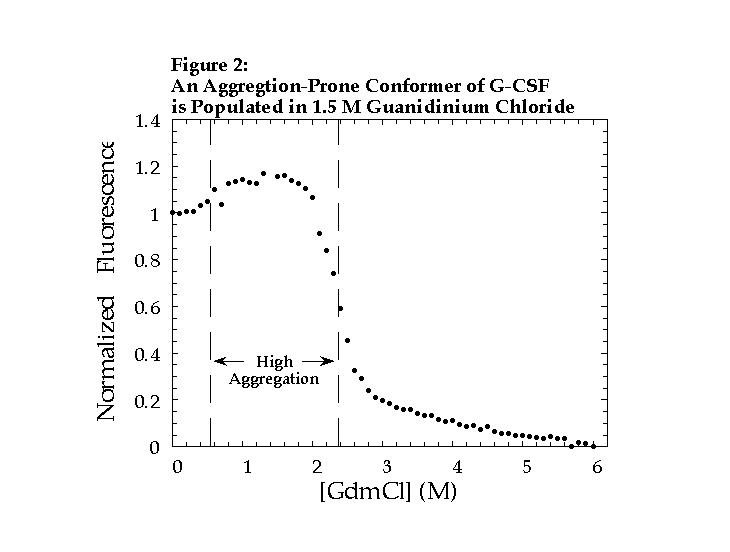
Equilibrium denaturation of G-CSF demonstrates that a highly fluorescent, aggregation-prone state is populated in 1.5 M GdmCl (Fig. 2). Fluorescence and CD analysis of tryptophan-substituted mutants indicates that a specific loop region, between the first and second helices, is perturbed in 1.5 M GdmCl. We propose that a similar conformational change occurs prior to aggregation under physiological conditions.
Current studies use quasi-elastic light scattering and Raman spectroscopy to focus on the global changes in secondary structure and formation of disulfide bonds as the aggregate grows in size. Combining these data, a comprehensive kinetic model of G-CSF aggregation is being developed.