Department of Chemical Engineering
In academic year 2003, the Department of Chemical Engineering maintained its usual high productivity and visibility in teaching and research. For the 14th consecutive year, our graduate program garnered the number-one ranking among the nation's chemical engineering departments by U.S. News and World Report. Our undergraduate program was also ranked as number one in the country by the same publication. The department also had a tremendous year fiscally, with research volume topping $24.6 million. This represents a 12.4 percent increase over last year's figure.
During the academic year, 28 doctoral degrees (PhD and ScD) were awarded, along with 33 SM and/or master's-level degrees, which total 61 advanced degrees conferred. Sixty-five SB degrees were conferred as of June 2003, with 49 percent being awarded to women.
The department's undergraduate enrollment stands at 170 students. The graduate student enrollment is stable at 281 students, with 245 in the doctoral program and 36 master's-level degree candidates. The graduate programs include 95 foreign students, 88 female students, and 50 self-identified minority students, of which 39 are Asian Americans. This year we received 328 applications for our doctoral program and offered admissions to 74 individuals, of whom 44 will be matriculating in the fall term of 2003.
 Walker Student Lounge, Building 66. Photograph © 2003 Walter Silver/Boston. |
Renovation of the common spaces and offices on the second floor of Building 66 has significantly enhanced the quality of life in the department. The key feature of the second floor is the Walker Student Lounge. Its bright and flexible new design has made this lounge a popular lunch spot, a place conducive to casual conversations between faculty, students, and staff throughout the day, and a great central location for departmental social events. The attractive new second floor office space aids in the recruitment of new graduate students and promotes interaction among them once they arrive for their first year on campus. In partnership with the Singapore-MIT Alliance (SMA) and the Dupont-MIT Alliance, the department has created a state-of-the-art videoconferencing facility on the third floor with the goal of enhancing long-distance educational opportunities and facilitating multi-institutional research collaboration. Finally, two new faculty offices and a conference room were created as a result of the second floor renovation. Laboratory space was renovated for two recent faculty hires: Professor Alice P. Gast and Professor Patrick S. Doyle.
The department hired one new junior faculty member this year: Dr. Kristala Jones Prather. Dr. Prather received an SB in chemical engineering from MIT and was winner of the Karl Taylor Compton Prize. She received a PhD in chemical engineering from the University of California, Berkeley before joining Merck, where she currently works in bioprocessing research. She will join the department in the fall of 2004. Her addition strengthens an already outstanding group of faculty in the important area of bioprocess technology.
We are very pleased to announce three faculty promotions effective July 1, 2003. Professor Gregory C. Rutledge was promoted from associate professor with tenure to full professor of Chemical Engineering. Additionally, we are delighted to announce that both Professor William H. Green Jr. and Professor Bernhardt L. Trout were promoted from assistant professor to associate professor without tenure.
The faculty has once again proven themselves leaders in their fields, and we are especially proud to note the following achievements of the past year. The department heartily congratulates Professor Gregory Stephanopoulos on his election to the National Academy of Engineering. The accompanying citation recognizes Professor Stephanopoulos for his pioneering contributions in defining and advancing metabolic engineering, in addition to his leadership in incorporating biology into chemical engineering research and education. Also in this past year, he was elected to a three-year term on the board of directors of the American Institute of Chemical Engineering (AIChE), continuing his record of longstanding service to the profession.
Emeritus Professor Edward W. Merrill received the coveted Founders Award of the Society for Biomaterials for 2003 for his landmark contributions to the discipline of biomaterials.
Professor Douglas A. Lauffenburger received the William H. Walker Award for Excellence in Contributions to the Chemical Engineering Literature, one of the most prestigious awards bestowed by the AIChE. Professor Lauffenburger was cited for his intellectual leadership in combining chemical engineering with molecular cellular biology and for using molecular and cell-level design principles in the development of innovative biotechnology applications.
We are delighted to have Professor George Stephanopoulos back in residence in the department after a two-year leave of absence, which allowed him to serve as chief technology officer of Mitsubishi Chemical. In this role, he spearheaded a significant restructuring of this company's research and development organization and set new directions that emphasized interdisciplinary strategic research.
Doctoral candidate Todd Zion and his team won MIT's renowned 50K Entrepreneurship Contest for their innovative work on SmartCells Technology for self-regulated delivery of insulin to the body. The basis for this idea evolved from his thesis work on nanoparticle systems with Professor Jackie Ying.
This year, the department was fortunate to welcome Ms. Melanie Miller, assistant to the department head; Ms. Alina Haverty, assistant to the executive officer; and Ms. Gwen Wilcox, administrative assistant to Professors Jefferson Tester and Bernhardt Trout. Additionally, we are delighted with the new appointments of Mr. James Hardsog in the position of systems administrator and Ms. Jean Belbin in the position of computer technical assistant.
Undergraduate Education
Undergraduate Enrollment over the Last Ten Years
| Class Level | 93–94 | 94–95 | 95–96 | 96–97 | 97–98 | 98–99 | 99–00 | 00–01 | 01–02 | 02–03 |
| Sophomores | 115 | 108 | 118 | 87 | 97 | 88 | 71 | 67 | 47 | 56 |
| Juniors | 90 | 104 | 101 | 121 | 90 | 90 | 85 | 76 | 66 | 49 |
| Seniors | 84 | 100 | 103 | 110 | 130 | 94 | 103 | 89 | 84 | 65 |
| Total | 289 | 312 | 322 | 318 | 317 | 272 | 259 | 232 | 197 | 170 |
Department undergraduate enrollment stands at 167 students. The trend toward reduced enrollment that we saw in the recent past seems to have reversed, with nearly a 20 percent increase in sophomore class size last year. The enrollment of women remains around 60 percent, and student quality remains excellent. We are preparing to offer a new degree in 10B chemical-biological engineering, which adds some fundamental biology subjects to the basic core of chemical engineering subjects. This degree is midway through the Institute approval process, and we anticipate full approval in fall 2003.
Graduate Education
Graduate Enrollment over the Last Ten Years
| Degree Level |
93–94 | 94–95 | 95–96 | 96–97 | 97–98 | 98–99 | 99–00 | 00–01 | 01–02 | 02–03 |
| Master's |
62 | 64 | 56 | 64 | 51 | 59 | 54 | 40 | 38 | 36 |
| Doctoral |
147 | 166 | 169 | 162 | 167 | 140 | 145 | 166 | 209 | 245 |
| Total |
209 | 230 | 225 | 226 | 218 | 199 | 199 | 206 | 247 | 281 |
Completing their second year of additional responsibilities are Professor Daniel Blankschtein as graduate officer and Professor K. Dane Wittrup as the head of the Graduate Admissions Committee. Because of their efforts in coordination with the faculty advisors of the department, 96 percent of the past year's rising class passed both the written and oral qualifying examinations and have thus been promoted to candidacy for the PhD/ScD. The incoming graduate class is entering with an average undergraduate GPA of 4.91/(5.0) and scored in the 88th percentile on the Graduate Record Examinations.
Faculty Notes
Professor Robert C. Armstrong continued as head of the Department of Chemical Engineering during the academic year. He has begun service as second vice chair of the Governing Board of the Council for Chemical Research (CCR); he will chair this organization in 2005. He has been working through CCR to organize a series of workshops to facilitate discussion broadly across chemical engineering regarding the evolution of the discipline. These discussions are driven by the significant impact of biology on research and education in chemical engineering today and by the breadth of industries into which our graduates now go. These meetings have led to a very interesting series of curriculum workshops funded by the National Science Foundation (NSF) to take a bold, in-depth look at the future of chemical engineering undergraduate education. These workshops are described in detail on the department's web site. Professor Armstrong currently serves on the External Advisory Boards and/or visiting committees of the respective departments of chemical engineering at the Georgia Institute of Technology, Northwestern and Texas A&M Universities, the Universities of Michigan at Ann Arbor and of Wisconsin at Madison, Tufts University, and the Virginia Polytechnic Institute.
Professor Daniel Blankschtein continued to serve as graduate officer, responsible for the educational and social well-being of 250 graduate students in the department. His research group conducts fundamental theoretical and experimental research in the area of colloid and surfactant science, with emphasis on practical and biomedical applications. Professor Blankschtein and his students presented papers at meetings of the American Institute of Chemical Engineers, the American Chemical Society (ACS), and the Society of Cosmetic Chemists, as well as at several industrial sites, including Alza Corporation, TransForm Pharmaceuticals, L'Oreal Paris, Firmenich, and Unilever Home and Personal Care USA. Professor Blankschtein's teaching responsibilities included 10.44J Statistical-Thermodynamics of Complex Liquids and 10.55 Colloid and Surfactant Science, a required core subject in the interdepartmental Program in Polymer Science and Technology (PPST). He continues to serve on the editorial board of Marcel Dekker's Surfactant Science series, and he received the New England Society of Cosmetic Chemists Award for the Best Research Paper in the Cosmetics Field, delivered to the society at a ceremony held in Boston.
Professor Charles Cooney returned from a year's sabbatical at the University of Cambridge and continues to serve as the faculty director of the Deshpande Center for Technological Innovation and codirector of the Program on the Pharmaceutical Industry. During the past year, he was a Cambridge-MIT Institute (CMI) fellow and co-taught the CMI pilot course, TEEMS (Technology-based Entrepreneurship for Engineering and Management Science Students), for engineering and management science undergraduates. Professor Cooney was appointed a coleader of the BP-MIT Projects Academy that was created by the Sloan School of Management and the School of Engineering.
Professor William M. Deen was an invited speaker at the World Congress of Nephrology in Berlin in June 2003 and (represented by one of his students, M. J. Lazzara) at the meeting of the European Society for Microcirculation in Exeter, UK, in August 2002. He gave invited lectures also at Oklahoma State University and Princeton University. Professor Deen received this year's Outstanding Faculty Award from the graduate students in the Department of Chemical Engineering. His group continued its investigations of physiological transport phenomena, which are supported by the National Institutes of Health. Their research topics centered on the hindered transport of macromolecules in biological hydrogels, water and protein filtration in kidney capillaries, and the reaction and diffusion of nitric oxide in cell culture systems used for toxicity studies.
Professor Patrick S. Doyle received a Career Award from the National Science Foundation to study the "dynamics of polymer collisions" using single molecule fluorescence microscopy and computer simulation. He was an invited lecturer at several meetings, including universities and hospitals. Among them were the Tufts University Department of Chemical and Biological Engineering, Massachusetts General Hospital, the New England Complex Fluids Meeting, and the Lab Automation Conference.
Professor Karen K. Gleason continued as executive officer for the department, coordinating revisions to the undergraduate curriculum and overseeing many space renovations, including the overhaul of the second floor of Building 66. Her group's work on chemical vapor deposition continues to result in new patents for biopassivation, dielectric, and optical coatings. Professor Gleason continues as chief scientific advisor for GVD Corporation, a start-up company she cofounded in 2001. Her invited presentations included talks at the ACS, Model Rocket Society, and AIChE conferences, seminars at the University of California, Los Angeles, Université de Montreal, and 3M. Her travels also included a "greening tour" at the Joint Readiness Training Center in Fort Polk, LA, sponsored by the Institute for Soldiers Nanotechnology.
Professor William H. Green Jr. was named associate editor of the International Journal of Chemical Kinetics in January. He chaired a session on Combustion Reaction Engineering at the AIChE national meeting and gave invited lectures at Wesleyan University and the Colorado School of Mines. With Professor Herb Sawin, he developed and taught the new subject 10.10 Introduction to Chemical Engineering. His research into computer methods for modeling complex chemical kinetics bore fruit, allowing for the construction of astonishingly accurate predictive models for pyrolysis systems and providing an improved conceptual understanding of high-efficiency, low-emission homogeneous-charge compression-ignition (HCCI) engines presented at the SAE World Congress in March. The computer models are being used to design more practical HCCI engines. William H. Green was promoted to associate professor.
Robert S. Langer is the Kenneth J. Germeshausen professor of chemical and biomedical engineering at MIT. Professor Langer has received over 100 major honors. In 2003 these included the John Fritz Medal (American Association of Engineering Societies), election to the Academy of Achievement, the Seymour J. Kreshover Lecture (National Institutes of Health), the Tripathy Endowed Memorial Lecture (University of Massachusetts, Lowell), the Skinner Memorial Lecture in Biomaterials (Northwestern University), the Maurice and Yetta Glicksman Lecture (Brown University), the Founders Lecture (University of Wisconsin), and the FMC Lecture (Princeton University). He was also presented with an honorary degree from the University of Liverpool, England, in 2003 and from the Hebrew University of Jerusalem in 2002. He was honored with the $500,000 Charles Stark Draper Prize, the world's most prestigious engineering prize, from the National Academy of Engineers in 2002. Professor Langer also received the Othmer Gold Medal (Chemical Heritage Foundation) and was the 2002 Distinguished Lecturer (University of Louisville) and the 2002 Institute Lecturer (American Institute of Chemical Engineers). In 2002, Forbes Magazine selected Professor Langer as one of the 15 innovators worldwide who will reinvent our future, and Discover Magazine named him one of 20 Biotech Geniuses to watch.
Professor Gregory Rutledge was promoted to full professor. He continues to serve in his role as director of the interdepartmental PPST at MIT and as editorial board member and special editor for Polymer and Computational and Theoretical Polymer Science. He taught a short course on Polymer Crystallization sponsored by the MESD division of AIChE. He delivered invited talks at the World Textile Conference, 6th World Congress of Theoretically Oriented Chemists, and the Asilomar Conference on Polymeric Materials, as well as at the University of Massachusetts at Amherst and Princeton University. He continues his research in the field of molecular engineering of soft matter through the development of molecular simulations, materials characterization, and nanofiber processing.
Professor Kenneth A. Smith has continued his research on the roles of fluid mechanics and transport phenomena in a number of contexts. These include use of the supercritical water oxidation process for destruction of organic wastes, a joint effort with Professor Jefferson W. Tester. With Professor T. Alan Hatton, he has been studying the dynamics of micellar self-assembly and the properties of a remarkable new class of photoresponsive surfactants. He is also engaged in the development of an instrument that can determine the size-segregated chemical composition of an aerosol and do so in real time. This has the potential of greatly enhancing our understanding of the origins, evolution, and dispersion of atmospheric contaminants that are found in the submicron particle size range. He is also participating in the Molecular Engineering of Biological and Chemical Systems program within the Singapore-MIT Alliance (SMA).
Professor George Stephanopoulos stepped down from his position as chief technology officer for the Mitsubishi Chemical Corporation's group of companies and resumed his full-time academic activities at MIT after a two-year leave of absence. He received an honorary doctorate in science from McMaster University and was elected as a member of the Board of the Mitsubishi Chemical Corporation. He presented lectures on the "Reformation and Rejuvenation of R&D in Mitsubishi Chemical" at the following conferences and symposia: the Institute for Research and Innovation, Japan; the Musashi Institute of Technology, Japan; the Center for Management of Technology, Japan; the Tokyo Institute of Technology; the Annual Meeting the Japanese Society of Chemical Engineers; and the Annual Meeting of the Korean Institute of Chemical Engineers.
This year, Professor Gregory Stephanopoulos was elected to the National Academy of Engineering. The citation made reference to his pioneering contributions in defining and advancing metabolic engineering as well as leadership in incorporating biology in chemical engineering research and education. Other honors include the Merck Award in Metabolic Engineering and election to the Board of Directors of AIChE. This past year, Professor Stephanopoulos gave a number of named lectures: the Fredrickson Lecture at the University of Minnesota, the Merck Lecture at the University of Virginia, the Patton Lecture at the University of Colorado, the J. M. Smith Lecture at the University of California at Davis, and the Kelly Lectures at Purdue University. He continued his educational and research activity in Bioinformatics and Metabolic Engineering. This program aims at the integration of genomic information and physiological data for the elucidation of cell physiology, leading to the rational modification and analysis of cell metabolism for medical and industrial applications. It comprises a research component as well as courses on metabolic engineering and bioinformatics. Professor Stephanopoulos became editor-in-chief of the journal Metabolic Engineering, published by Elsevier. In addition, he continued to serve on the editorial boards of seven other scientific journals.
He delivered plenary lectures at the University of Ioannina, Greece (March 2002), at the Conference on the New Biology at the University of Illinois (March 2002), at the Discrete Mathematics and Theoretical Computer Science Center Conference at Rutgers University (April 2002), the European Symposium of Applied Biocatalysis (Como, Italy, May 2002), the DECHEMA Annual Biotechnology Meeting (Wiesbaden, Germany, June 2002), the International Conference of Bacteriology and Applied Microbiology (Paris, August 2002), and the American Academy of Arts and Sciences Annual Meeting (Denver, February 2003). At MIT, he contributed to the proposed 10-B Chemical-Biological Engineering curriculum development.
Professor Jefferson W. Tester continues to be active in the energy and environmental area, where he coordinates technology-related programs for the Laboratory of Energy and the Environment. He is the chair of the National Advisory Council of the Department of Energy's (DOE) National Renewable Energy Laboratory and cochair of the Governor's Advisory Board for the Massachusetts Renewable Energy Trust. Professor Tester also continued as a member of the advisory groups for the Paul Scherrer Institute, which is part of the Eidgenössische Technische Hochschule (ETH/Swiss Federal Institute of Technology) in Zürich, Switzerland; the Nuclear and Energy Systems Division of the Idaho National Engineering and Environmental Laboratory (INEEL); and the Division of Earth and Environmental Sciences at the Los Alamos National Laboratory. Last year he served on a National Research Council committee that reviewed the US concentrating solar energy program and cochaired the New England Regional Conference on Hydrogen Infrastructure. He gave invited lectures at ETH in Zürich, Ecole Polytechnique Fédérale de Lausanne (Switzerland), the University of Rochester, Enitechnologie in Milan, the State University of New York, the University of Utah, the Los Alamos National Laboratory, the John Deere Company, and Statoil in Trondheim, Norway. A new textbook on sustainable energy coauthored by Professor Tester and others at MIT has been completed.
Professor Bernhardt L. Trout was promoted to associate professor. He has given invited talks at Stanford University, Princeton University, and many other engineering institutes, in addition to AIChE and ACS. Funding for his projects on chemical and process design via molecular understanding is provided by NSF, DOE, the National Aeronautics and Space Administration, the Government of Singapore, Merck, Inc., and Amgen, Inc.
Professor Daniel I. C. Wang was the keynote lecturer at the "Trend in Biotechnology" Conference held in Vienna, Austria, in November 2002. He also presented a series of lectures at the Department of Biochemical Engineering, Catholic University, in Valparaiso, Chile, in March 2003, where he was bestowed an honorary doctoral degree in biochemical engineering. He presented a keynote lecture at the Bayer's Strategic Research and Development Retreat in April 2003 at Beaufort, NC. He was invited by the Ministry of Education, People's Republic of China, to deliver a series of lectures in biochemical engineering and biotechnology at Tsinghua University, Beijing, in June 2003.
Professor Wang continues as a faculty fellow in the Singapore-MIT Alliance program, in which he delivered his course, Bioprocess Engineering, at the National University of Singapore in January and March 2003. In addition, Professor Wang is the coadvisor to three PhD candidates in the SMA program. During 2002 and 2003, Professor Wang has been in charge of presenting a new laboratory Course 10.28 Biological Engineering Laboratory as part of the department's curriculum for the new Course 10-B.
Professor Jackie Y. Ying delivered 11 invited lectures at various international conferences and national meetings during the past year, including plenary lectures at the 3rd International Symposium on Mesostructured Materials in Korea, the 4th World Congress on Particle Technology in Australia, the 17th International Symposium on Chemical Reaction Engineering, and the Nanomaterials Crossroads Symposium. She served on the Scientific/Programming Committee of the 6th International Conference on Nanostructured Materials and on the Organizing Committee of the 2nd Japan-America Frontiers of Engineering Symposium. Professor Ying was an invited seminar speaker at the University of Queensland (Australia), Kansas State University, Stanford University, and Columbia University. She serves on the editorial boards of eight journals/book series, as well as on the advisory boards of the Leibniz-Institut für Festkörper- und Werkstoffforschung, Dresden (Germany), the University of Queensland Nanomaterials Centre (Australia), the National Research Council Steacie Institute for Molecular Sciences (Canada), and the Institute of Materials Research and Engineering (Singapore). Professor Ying is an honorary professor of chemistry of Jilin University (China) and serves on the Canvassing Committee of the American Chemical Society Award for Creative Research in Homogeneous or Heterogeneous Catalysis. She chaired the SMA program on Molecular Engineering of Biological and Chemical Systems. Professor Ying is currently on professional leave in Singapore as the executive director of the Institute of Bioengineering and Nanotechnology, a new national research institute under the Agency of Science, Technology and Research.
Research Highlights
Toxicity of Endogenous Nitric Oxide (Professor William M. Deen)
Researchers in numerous branches of medicine and physiology have discovered in recent years that nitric oxide (NO) is routinely synthesized by cells throughout the body and that maintaining proper concentrations of it is essential for good health. It helps regulate blood pressure, affects clotting, assists the communication among neurons, and plays a role in the immune response to infections, among other beneficial actions. But there can be too much of a good thing. The problem is that NO is both toxic and mutagenic if present in excess, and there are situations in which its production is apparently overstimulated. The sustained, high rate of NO synthesis by immune cells during chronic inflammations provides an example, and this may explain the statistical link between persistent inflammations and certain forms of cancer. Chemical damage to cellular proteins, lipids, and DNA results not so much from NO itself but from a variety of trace nitrogen oxides formed when NO is oxidized in biological fluids.
Professor Deen's group has been investigating fundamental aspects of the production and fate of NO and related nitrogen oxides in biological systems in collaboration with several Faculty in the Biological Engineering Division. To assess health risks and design interventions, one needs to know which compounds actually mediate the various harmful effects of excess NO. Underlying that effort is the need to know what their concentrations are near an NO source (e.g., an activated macrophage) and over what distances those concentrations remain elevated. A chemical engineering problem emerges: the concentration fields must reflect the interplay between rates of reaction and diffusion. The modeling problems are made challenging by the complex chemistry, which includes enzymatic sources and sinks for NO, a network of inorganic oxidations that yield other reactive nitrogen oxides, and the interactions of the nitrogen oxides with soluble or structural biomolecules. The geometric complexity of tissues—and even of cell culture systems used to study NO toxicity—adds its own difficulties. Experimentally, few of the compounds of interest are present at measurable concentrations, and their levels must be inferred from analyses of oxidation end products and of biomarkers (e.g., trace levels of nitrogen oxide–modified proteins).
This work in Professor Deen's group has led to several recent insights. A reaction-diffusion model developed to describe the fate of NO released by macrophages cultured on plates revealed that some species will be present only in the immediate vicinity of the cells, but others will exist throughout the culture medium. Accordingly, various chemical reactions will be spatially segregated, even in extra-cellular fluid. Experiments varying the depth of the liquid medium indicated that NO strongly inhibits its own synthesis by macrophages, a phenomenon that had been demonstrated previously with isolated enzymes but not with intact cells. An analysis of the kinetics of NO production and consumption in macrophages suggested that the maximum NO concentration that can be achieved at any cell number density is about 1 μM. This was the first indication of a possible upper limit for NO concentrations at sites of inflammation. Knowing better what to mimic, an apparatus was designed to permit "target cells" (cells that do not produce NO) to be exposed to controlled, micromolar levels of NO for up to several days. In collaboration with Professor Gerald N. Wogan of the Biological Engineering Division, the effects of NO concentration and total dose (area under the concentration-time curve) were then characterized in terms of cell survival and several types of cellular damage. It was found that there are dual thresholds for NO toxicity. Toxic effects were not seen unless a minimum concentration and a minimum dose were both exceeded. The concentration threshold for the cell lines used was about 0.5 μM (about half the inferred physiological maximum). This is the first quantitative demonstration of toxicity thresholds for NO, and it is stimulating a new round of experiments to examine the underlying intracellular events. An important objective in the next few years is to develop reaction-diffusion models for tissues that will provide a rational means to extrapolate kinetic, diffusional, and cell culture data to pathophysiological conditions in the body.
Two-Dimensional Protein Crystals: Physics Lessons from Nature (Alice P. Gast)
The surfaces of many prokaryotic cells (bacteria and archae) are fully enveloped with an organized layer of proteins. The purpose of these ordered protein coats is not fully understood, although it is postulated that they play a role in the physical integrity and protection of the cell or in sieving materials passing through the membrane. These fascinating structures in nature motivate the Gast group to study model systems of proteins on lipid monolayers or bilayers. In addition to the interesting, naturally occurring assemblies, ordered arrays of proteins on lipid supports are a useful system to study protein structure and interactions. Lipid monolayers and bilayer membranes serve as simplified models to the complex and inhomogeneous properties of actual cell membranes. Appropriately cloaked liposomes also have potential uses as carriers for targeted drug delivery.
Several years ago we began studying a model system—streptavidin—a tetrameric globular protein having an unusually high binding affinity for biotin. We studied the crystallization of streptavidin attached to lipid monolayers spread at the air-water interface. By changing the solution pH, we were able to provoke different crystalline symmetries and shapes due to the changing intermolecular interactions between proteins. We tested our understanding of these molecular interactions by creating point mutations that altered the surface of the protein-enhancing attractions or repulsions and driving the crystallization behavior. The ability to alter single amino acids in this way provides a powerful link to molecular models of protein-protein interactions. These studies have enabled us to learn much about the two-dimensional crystallization process and how we can tailor lattice structure and crystal morphology through manipulating intermolecular forces.
Our interest in naturally occurring protein crystals such as those enveloping archae led us to create giant unilamellar vesicles as model membranes. We were able to study the same crystallization phenomena on these vesicles of about 50–100 micrometers in diameter through confocal fluorescence microscopy. We find that crystals grow on these surfaces even under conditions of very low ionic strength and in the presence of sugar solutions. A typical series of images shows fluorescently labeled background protein surrounding dark regions of streptavidin crystals.
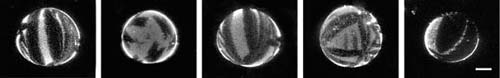 Streptavidin crystals. |
We collaborated with R. Merkel in the Sackmann group in Munich, Germany, to study the effect of a surface crystalline protein layer on the mechanical properties of the membrane. In particular, we used micropipette aspiration to probe the membrane responses and material properties of bare vesicles and those coated with either an amorphous or a crystalline protein layer. The presence of crystals on the vesicle surface transformed them into roughened spheres and prolate ellipsoids. The coated vesicles have a plastic response to deformation as seen in the structure set by aspiration with a pipette shown below. The composite protein-lipid membrane combines the solid characteristics of two-dimensional crystals with the fluid elastic behavior of lipid bilayers to yield a thin, rigid, yet viscoelastic structure. The nature of the protein-lipid and protein-protein interactions plays a key role in determining the overall properties of the composite membrane.
 Coated vesicles. |
Crystallizing proteins on giant bilayer vesicles provides us with a well-defined system that is easy to study and manipulate. The unique properties of these structures facilitate a fundamental understanding of molecular self-assembly and protein-membrane interactions.
Therapeutic proteins encompass a significant and growing fraction of the pharmaceutical market. An example is Amgen's G-CSF, which has annual sales of $2 billion and is used to treat complications arising from chemotherapy. It is certainly true that designing therapeutic proteins like G-CSF and processes to manufacture them are great accomplishments. But they are not enough. In order for therapeutic proteins to be useful and to be approved by the FDA, they must be stable for two years in a form that can be conveniently and safely injected into a patient. Typically, this form is an aqueous solution consisting of water and a variety of cosolutes, such as salts, sugars, surfactants, amino acids, and others.
The major problem in developing the solution formulation is that no one knows how to do it for a particular protein, aside from using heuristics and trial-and-error experimentation. This Edisonian approach has failed in many cases, so that there are numerous therapeutic proteins that have the potential to save lives and enhance the quality of lives but that cannot be used because they are not stable in storage. Furthermore, in cases where the Edisonian approach succeeds, it does so after many experiments, the number of which would be sharply reduced if the underlying chemical processes involved in destabilization and stabilization were understood. A major reason why they are not understood is that it is almost impossible using current experimental technology to isolate the individual interactions and reactions among chemical species that lead to destabilization or stabilization. This is because many trillions (actually, roughly 1023) of chemical processes are occurring during any single experiment and multiple very clever, very accurate experiments would need to be performed in order to isolate the chemical processes of interest. On the other hand, accurate mathematical models of the systems would allow the study of the most important chemical events isolated from the less important ones.
The Trout group is thus developing and solving mathematical models for the design of solutions that will stabilize therapeutic proteins against oxidation and aggregation, the two most common routes of degradation of therapeutic proteins. For these models to be accurate, they must include each atom explicitly—and for the most important regions of the protein and solution, each electron explicitly. The latter is essential for the accurate treatment of the breaking and formation of chemical bonds and requires the use of approaches based on quantum mechanics.
Having an accurate model is not enough. This is because the models that we develop are exceedingly complex, and we must devise clever ways of extracting the information that we want from these models efficiently and with a minimum of numerical noise. In order to accomplish this task, we use sophisticated simulation approaches, some of which are developed in our laboratory. These approaches are based on statistical mechanics, which gives us physical insight into which numerical regions of our model we should focus on. Even using these statistical mechanical methods, each data point takes more than a billion trillion (1015) computations. Together with these computational approaches, we are performing experiments on well-defined model systems in order to validate our theoretical/computational approaches.
In terms of specific results, we have isolated the essential reaction pathway that leads to the oxidation of sulfur sites on methionine residues. Furthermore, we have demonstrated that the rate of oxidation is proportional to the number of water molecules in the vicinity of the sulfur site. Tersely put, if one can keep water away from the sulfur sites, even with a large amount of oxidizing components present in the system, the sulfur will not be oxidized. We are currently designing chemicals—called excipients—that selectively surround those sulfur sites and block them from water. Our computational modeling approach gives us unique insight into what is happening at the sulfur sites and what properties of the excipients are necessary so that the excipient surrounds the target site selectively.
In the area of aggregation, we have validated our models for the computation of the change in stability of proteins, such as RNase, in the presence of sugars, polyols, and urea. We have also developed a quantitative theory of what the essential properties of an excipient are that makes it a stabilizer. Using this theory, we are designing new molecules that should function better than currently used excipients, and we are developing computational methods to aid in the design of these new molecules and experimental protocols to test them.
Annual Lectures, Seminars, and Symposium
The department once again hosted a very successful series of four major annual lectures: the 4th Frontiers in Biotechnology Lecture, delivered by William H. Rastetter, chairman and chief executive officer of IDEC Pharmaceuticals Corporation; the 16th Hoyt C. Hottel Lecture, delivered by John H. Seinfeld from the Department of Chemical Engineering at the California Institute of Technology; the 8th Alan S. Michaels Lecture, delivered by Leroy Hood, president and director of the Institute for Systems Biology; and the 25th Warren K. Lewis Lecture, delivered by Nance Dicciani, president and chief executive officer of Specialty Materials at Honeywell International, Inc.
Our Departmental Seminar Series featured academic and industry leaders from Princeton University, Stanford University, the University of Colorado, the University of Illinois–Urbana, the California Institute of Technology, the University of Massachusetts–Amherst, the University of Pittsburgh, the University of California–Berkeley, the University of Toronto, and the University of Michigan.
On September 27, 2002, a symposium in honor of Jack B. Howard was held, with speakers from the University of Massachusetts–Amherst, the University of Utah, Brown University, the Colorado School of Mines, the University of California–Los Angeles, and the highly respected industrial organization Advanced Fuel Research, Inc.
Departmental Awards
The Department Awards Ceremony took place on May 12, 2003, in the Gilliland Auditorium of the Ralph Landau Building. We are pleased to recognize this year's recipients of the Outstanding Faculty Awards: Professor William M. Deen was chosen by the graduate students and Dr. C. Michael Mohr was honored by the undergraduate students.
The Edward W. Merrill Outstanding Teaching Assistant Award was presented to third-year graduate student Paul Yelvington for his work in 10.10 Introduction to Chemical Engineering.
Chemical Engineering Special Service Awards were conferred to the members of the Graduate Student Council, Bradley Cicciarelli, Anna Pisania, Keith Tyo, Ramin Haghgooie, and Daryl Powers, and to Bradley and Ramin for service in organizing intramural sports. In addition, Bradley Cicciarelli was awarded the "Chemical Engineering Rock" for outstanding athleticism. All third-year graduate students are required to present a seminar on the progress of their research, and the two recipients of the award for outstanding seminar delivered were Paul Yelvington and James Bielenberg.
Our undergraduates also earned numerous accolades over the course of the year. Sudha Amarnath received the Dow Chemical Company Outstanding Junior Award for excellence in balancing academics, social and professional organizational commitments, and work experience. Junior Amy Shi and sophomore Veronica Andrews were each awarded a Merck Fellowship. The Robert T. Haslam Cup, which recognizes outstanding professional promise in chemical engineering, went to Geoffrey Von Maltzahn. Finally, the Roger de Friez Hunneman Prize, the oldest prize in the department, awarded to the undergraduate who has demonstrated outstanding achievement in both scholarship and research, went to Philip Lee.
The department is quite pleased to recognize Mr. Brett Roth, assistant to Professors Charles Cooney and Gregory Stephanopoulos, as its Outstanding Employee of the Year. Mr. Roth was elected by his peers and the graduate students for having provided unrivaled dedication and outstanding service to Faculty, staff, and students. Mr. Stephen Wetzel, facilities coordinator, was awarded the Individual Accomplishment Citation for his dedication to the department and hard work on behalf of the graduate students, Faculty, and staff that work in Building 66.
The Department of Chemical Engineering has had a very fruitful and rewarding year in 2002–2003, and it is poised for even bigger and greater successes in the upcoming year.
More information about the Department of Chemical Engineering can be found on the web at http://web.mit.edu/cheme/.