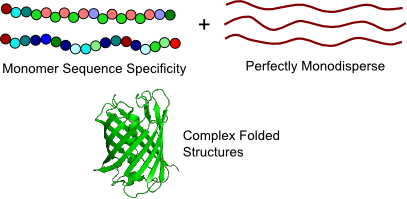
Despite great advances in controlled polymerization techniques, synthetic polymerizations produce materials characterized by polydispersity in molecular weight and by relatively simple monomer sequences, such as random and block copolymers. In contrast, the biological synthesis of proteins allows us to produce polymers that are perfectly monodisperse with very reliable control over the chemical identity of each individual monomer. This exquisite degree of chemical control makes proteins ideal models for understanding complex problems in polymer physics. Their sequence specificity leads to complex hierarchical structures (protein folds) and specific interactions that may affect protein self-assembly.
Proteins as Polymers
Synthetic Polymers
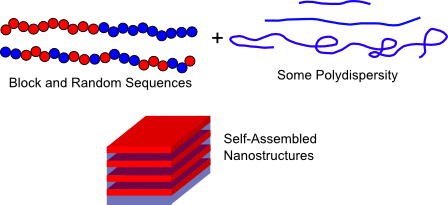
For example, α-helical proteins are model rodlike polymers that can be used to investigate liquid crystallinity in macromolecules or about the effect of chain stiffness on block copolymer self-assembly. Alternately, crystalline ordering of β-sheets provides a model for polymer crystallization. Control over protein interaction strength and multiplicity allows physically associating protein gels to be engineered with structural control similar to what could only be previously achieved in chemical gels.
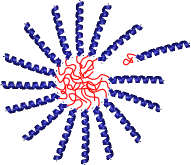
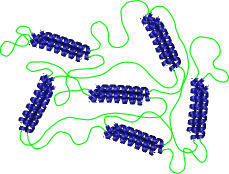
Helix-coil proteins forming a micelle and a gel from telechelic proteins.