SIR2 and aging: an historical perspective
SIR2 was originally identified as a factor necessary for transcriptional
silencing at the silent mating loci and telomeres. SIR2 functions at
these loci in a complex with SIR3 and SIR4. More recently, a role
for SIR2 in silencing at the rDNA has been demonstrated. Presumably,
it is the ability of SIR2 to establish a more closed chromatin structure
at the rDNA that accounts for its anti-recombinogenic properties at this locus.
The discovery by Matt Kaeberlein and Mitch McVey that overexpression of SIR2
is sufficient to extend life span in yeast promoted SIR2 to the forefront
of aging research in this organism.
Until recently, the catalytic function of SIR2 was unknown. Based on
the ability of Sir complex to promote silencing, it had been speculated that
SIR2 might act as a histone deacetylase; however, many groups attempted
and failed to detect this activity in vitro. In late 1999, the Moazed
lab published that SIR2 has a weak ADP-ribosyltransferase activity. Shin
Imai and Chris Armstrong had independently discovered the ADP-ribosyltransferase
activity of SIR2 and were working to characterize this activity further
when they discovered a shocking surprise. SIR2 is also a histone deacetylase!
Not only that, but it is a novel kind of histone deacetylase in that it requires
NAD to function. Furthermore, the histone deacetylase activity of SIR2
is required for all of its in vivo functions, including promoting longevity.
At about the same time, Suju Lin and Pierre Defossez were studying life span
extension in yeast by caloric restriction. They had found that growing the mother
cells in low glucose (0.5% rather than the normal 2%) resulted in an increased
life span. This life span extension was mediated by decreased cAMP and, thus,
lowered protein kinase A signalling. How caloric restriction and decreased cAMP
were able to extend life span was not understood.
|
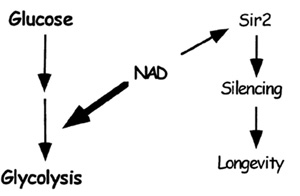
The discovery that Sir2p requires NAD for its activity immediately suggested
a link between SIR2 activity and caloric restriction. This link
was strengthened by the observation that life span extension by caloric
restriction requires Sir2 protein. Caloric restriction is likely to reduce
the carbon flow through glycolysis and result in more free cytoplasmic
NAD. SIR2 could act as a sensor of NAD levels within the nucleus.
Under conditions of caloric restriction, NAD levels are high, SIR2
is activated, and the rate of aging is decreased. |
Recently, Heidi Tissenbaum made the startling discovery that overexpression
of a C. elegans Sir2p homolog is also able to extend life span that
organism. This is particularly surprising because the C. elegans adult
life span is completely post-mitotic. This results suggests that chromatin structure
and/or NAD levels might be relevant for life span regulation in higher organisms,
including humans.
We speculate that life span extension by caloric restriction in mammals may
act by increasing the activity of SIR2 proteins. Recent work by Suju Lin has
demonstrated that caloric restriction of yeast increases Sir2p activity by increasing
respiration and consequently increasing the cellular NAD/NADH ratio. We are
currently pursuing research projects in mice and humans to address the possibility
that this mechanism of Sir2 regulation is conserved.


