Research Interests
- Computational solid mechanics
- Modeling of coupled electrochemical-mechanical systems
- Energy storage materials modeling
- Fracture mechanics
- Coarse grained modeling of polymers

|
| MIT postdoctoral associate Giovanna Bucci developed modeling tools to explore the complex mechanical and electrochemical interactions of lithium ions with battery materials. Mechanical stress is a significant force in batteries made of all solid materials, causing separation of electrolytes from electrodes and causing individual particles of battery materials to become isolated and unable to effectively transfer energy, leading to deteriorating battery performance, and eventually to battery failure. Photo, Denis Paiste, Materials Processing Center. |
To overcome the danger of fires and increase the energy density of lithium batteries, researchers are developing solid-state lithium batteries that replace flammable liquid electrolytes with a safer solid electrolyte. These solid-state lithium batteries are like a tightly connected sandwich of cathode, electrolyte and anode, and a common problem is separation of these layers from each other and mechanical degradation within the layers, which leads to weaker battery performance and eventual failure. "In particular, for all solid-state batteries, the mechanical degradation is very significant for the durability of the battery," explains MIT postdoctoral associate Giovanna Bucci.
The mechanisms that lead to this failure are a complex mix of electrical, chemical and mechanical factors. They affect the capability to transfer lithium ions back and forth through the electrolyte to the cathode and anode. With a strong background in solid mechanics, Bucci developed non-linear continuum mechanics-based simulations, using finite element analysis and writing computer code, primarily in C++, to model these interactions.
Mechanical and Electrochemical Coupling
"Generally speaking, my work is to analyze the coupling between mechanical and electrochemical effects," she says. Bucci's expertise in continuum scale simulation enables her to model mechanical and chemical behavior of battery charging and discharging on a suitable time scale and cell size. "It is not possible to do it with atomistic calculation because they cover a very small range of time and space," she says. The overall goal of the research is to find the combination of material properties and microstructure engineering that is most failure tolerant. The design of a battery considers the geometry of the microstructure (particle size and distribution), and the operating conditions, in other words how fast the battery is charged and discharged.
Bucci, who received her PhD in Italy and previously was a postdoctoral associate at Brown University, started working on the project under MIT Professors W. Craig Carter and Yet-Ming Chiang in March 2014. She also is working on solid oxide fuel cells under Professors Harry Tuller and Bilge Yildiz, with funding from the COFFEI project (DOE grant DE-SC0002633).
Her current MIT post is Bucci's first experience in a materials science department. Professor Carter says of Bucci, "She has an incredible background in theoretical mechanics, which you wouldn't expect would have very much application in materials science. But, she's been able to bridge the gap between this rigorous foundation, learning some of the elements of materials science and putting those together in a way, which, I think, has not been done before. She's writing up some very fundamental work right now and then doing some simulations, which shed light on the behavior of battery microstructures and solid oxide fuel cells. I'm very pleased with her work."
Computer Animations
From her work, Bucci has created animations that show the swelling associated with lithium ions flowing into the cathode or anode material, which is called intercalation. The swelling causes mechanical stress on the material, which can lead to incomplete absorption of available lithium ions and lessen a battery’s ability to hold a charge.
| An animation by MIT postdoctoral associate Giovanna Bucci shows that electrode particles swell, and stress rises, as lithium ions flow into the particles in a simulation of a solid-state lithium ion battery. The swelling generates compressive stress in the particles. As the stress increases, the diffusion (or intercalation) of lithium becomes slower and slower. Courtesy of Giovanni Bucci. |
"During cycling, the particles undergo some volumetric change, some swelling and deswelling, because lithium is intercalating in the particles, and so the interface between the two solid materials can delaminate because of this volume change and the mechanical stress that arises from that," Bucci explains. "This means that some of the particles can be disconnected from the electrolyte. Since the electrolyte will carry the lithium ion, that could be an increase in time for charging the battery; there also could be capacity loss, because an electrode particle becomes completely isolated, so lithium cannot reach some of those particles."
Swelling, Stress Cause Fractures
Stress is an important problem that cannot be ignored, she says. Batteries opened after they have been charged and discharged many times show fractures in particles of electrode material that develop because of this volume change and the stress that accompanies it.
When lithium diffuses into electrodes (the active materials of a battery), the material has to expand like a sponge to absorb it. But in a solid-state battery, the electrode particles are constrained by the surrounding solid matrix, and their capability to absorb lithium is limited, Bucci says.
"The material swells but also this swelling means that the lattice is stressed; so there is mechanical stress there, and then this mechanical stress affects the diffusion of lithium, so the coupling goes in two directions," she adds. Most cathode and anode materials undergo some change of volume, which could be from a few percent to a very large change. Silicon, for example, can undergo a very large volume change up to 300 percent, while compounds that are commonly used as a cathode or anode material undergo between 5 and 10 percent volume change. Even moderate volume changes have a large impact on the overall battery performance, she says.
This lithium flow, or flux, is made harder by the presence of compressive stress. Bucci's work also shows that inhomogeneous mechanical stress causes lithium to concentrate in areas of higher tensile stress and migrate away from regions where the material is compressed. A related computer animation shows this effect. "It creates a stress gradient across the sample and demonstrates how the stress, in turn, drives lithium diffusion," she explains. "The particles are usually solid cathodes and anodes, but in the case of the solid electrolyte, the effect is bigger because the system is more constrained. If there is a liquid or a very soft material in between the particles, the system is more tolerant to deformation."
| An animation by MIT postdoctoral associate Giovanna Bucci emphasizes the role of mechanical stress as a driving force for diffusion. Stress-concentration can be observed in electrode particles, for instance around a crack tip or close to sharp corners. Stress gradient drives lithium to localize in such regions, where a higher tensile stress induces the lattice to expand and better accommodate lithium intercalation. Courtesy of Giovanna Bucci. |
Selective Carriers
Despite posing some challenges compared to liquid electrolytes, solid electrolytes have a different advantage of being selective carriers for lithium. A selective solid electrolyte suppresses unwanted side reactions, which a liquid electrolyte would allow. "The solid electrolyte, if it has been engineered for being a lithium ion carrier, it will just do that. It will allow us to use a combination of cathode and anode materials that would be unstable with liquid electrolyte," Bucci says.
This could allow for batteries with higher energy density, based on a combination of anodes and cathodes that cannot currently be used with liquid electrolyte. Since solid electrodes can be extremely thin, multiple battery cells can be stacked to produce higher voltages, for example, for an electric car, unlike organic liquid electrolyte, for which every cell needs to be singly packaged and then connected externally. "The cells can be stuck one on top of the other without being separated from each other, and so it reduces the volume, and the mass, of the overall system," she notes.
Diffusion Issue
Lithium ion flux tends to be slower in solid electrolytes, but research continues to find solid electrolytes that carry lithium ions faster.
As particles absorb lithium ions, swelling can lead to the loss of contact between electrode particles and electrolyte. "The mechanical degradation of the system is strongly connected to the durability of the battery. The mechanical characteristics of the solid electrolyte are very important. Because if it is a soft material, it will allow this volume change more easily than if it is a rigid material, or a brittle material, that can easily crack," Bucci explains. Her animation shows that, if the particle is connected completely to the electrolyte, lithium ions cross into the electrode particle and reach its core. But having some of the interfaces delaminated means that lithium cannot flow across the interface and charging of the battery is slower because the lithium ion has to follow a more difficult path to reach the particle.
Solid-state lithium batteries at large scale have potential for electric cars, while miniaturized ones can power medical devices. For thin film batteries, with anodes, electrolyte and cathodes on top of each other, the electrolyte can be less conductive and still make an efficient battery because it is so thin, Bucci suggests.
One solid electrolyte, LiPON (lithium phosphorus oxynitride), was developed at Oak Ridge National Lab, and it has been primarily tested in thin film batteries. Researchers there published a study in October 2014 showing a 5-volt solid-state battery with a lifetime of more than 27 years with a daily charge/discharge cycle. Japanese researchers had recent success with several different initiatives involving lithium ion batteries. A 2014 paper by researchers at Tokyo Institute of Technology and Toyota Motor Corp. analyzed ionic conductivity of solid electrolytes made from crystals of Li10GeP2S12 with varying combinations of silicon or tin. Professor Ryoji Kanno and Associate Professor Masaaki Hirayama, Electronic Chemistry, and their team won a U.S. Patent on April 15, 2014, for a high-output solid-state battery with a sulfide solid electrolyte and excellent ion conductivity. Hitoshi Takamura, Professor of Energy Materials at Tohoku University, demonstrated in a May 2014 paper that a "parasitic conduction mechanism" enhanced lithium ionic conductivity in a solid electrolyte composed of lithium borohydride (LiBH4) doped with potassium iodide (KI).
In a series of papers, a group led by Masahiro Tatsumisago, Professor of Applied Chemistry at Osaka Prefecture University, demonstrated that the glass-ceramic material Li2S-P2S5 works well as a solid electrolyte for solid-state lithium batteries with high lithium-ion conductivity and reversible capacity. Using solution processing, the researchers coated the glass-ceramic material onto LiCoO2 particles. Quality of the contact at the interface between a solid electrode and a solid electrolyte is a key to improving battery performance, Tatsumisago and co-authors noted in a review article, "Recent development of sulfide solid electrolytes and interfacial modification for all-solid-state rechargeable lithium batteries," in the Journal of Asian Ceramic Societies.
| A non-linear continuum mechanics-based simulation by MIT postdoctoral associate Giovanna Bucci shows that as cracking occurs in a solid-state battery system some particles of the electrode and electrolyte become partially disconnected, or completely isolated, and so can no longer take in lithium ions, leading to lesser battery performance. The white lines are diffusion fronts, points characterized by the same lithium concentration. Particles that are disconnected to some extent from the electrolyte cannot be uniformly lithiated, so they appear in part rust colored and in part gray colored. Courtesy of Giovanna Bucci. |
Bucci's work made use of NSF computational resources through the XSEDE network. "The heavy calculation is split up among many processors and that will speed up the time of the whole simulation, and it allows us to scale up the problem, so going from tens of particles to a system of hundreds particles or a thousand (electrode particles)," she explains. The problems her coding addresses are not easy to solve because of the coupling of different physical phenomena. "I have a series of equations that need to be solved simultaneously, and this requires appropriate computational tools," she says. Developing the main structure of her code took about six months, and she adds new features as needed. Her work is still unpublished, but she is working on a manuscript. Bucci gave a presentation on April 15, 2015, at MIT at the Rising Stars in Nuclear Science and Engineering symposium, which features promising women researchers.
Bucci's initial model featured square particles in a matrix because fractures are more likely to initiate at sharp corners. "People usually do a representation of spherical particles, and try to understand what happens when you lithiate, and how big the stress is and try to go from there and extend to a real cell," Bucci says. "In this case, we can directly model a complex electrode-electrolyte system and see if it fractures or it doesn't, and if it fractures, what consequences that would have. So, I think in that sense, it's a unique tool that we have." Her next step will be to import data from X-ray tomographic microscopy images of real battery microstructures by making use of OOF3D, a freely available library developed at the National Institute of Standards and Technology.
Being able to simulate a realistic battery system would have a large impact in guiding the experimentation towards promising solutions.
Bucci was born on a farm in southern Italy. "My father is a mason and my mother is a dressmaker. I didn't know that I would end up being a research scientist and working at MIT. It's very far from what I could see at the time." She got her undergraduate degree in architecture at Università degli studi di Pavia in Milan before earning her doctorate in Structural Engineering Department at Politecnico di Milano.
Bucci is married to MIT aeronautics and astronautics graduate student Brandon L. Talamini, and their daughter, Iolanda, was born May 9, 2015. "We share a lot of interests in the research that we do," she says. Bucci hopes to begin looking for a faculty position in the fall.
– Written by Denis Paiste, Materials Processing Center
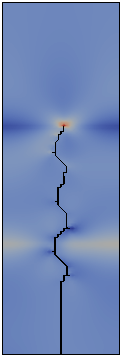
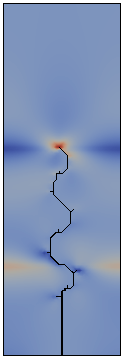
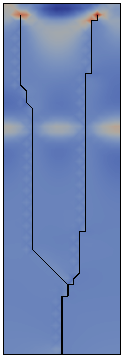
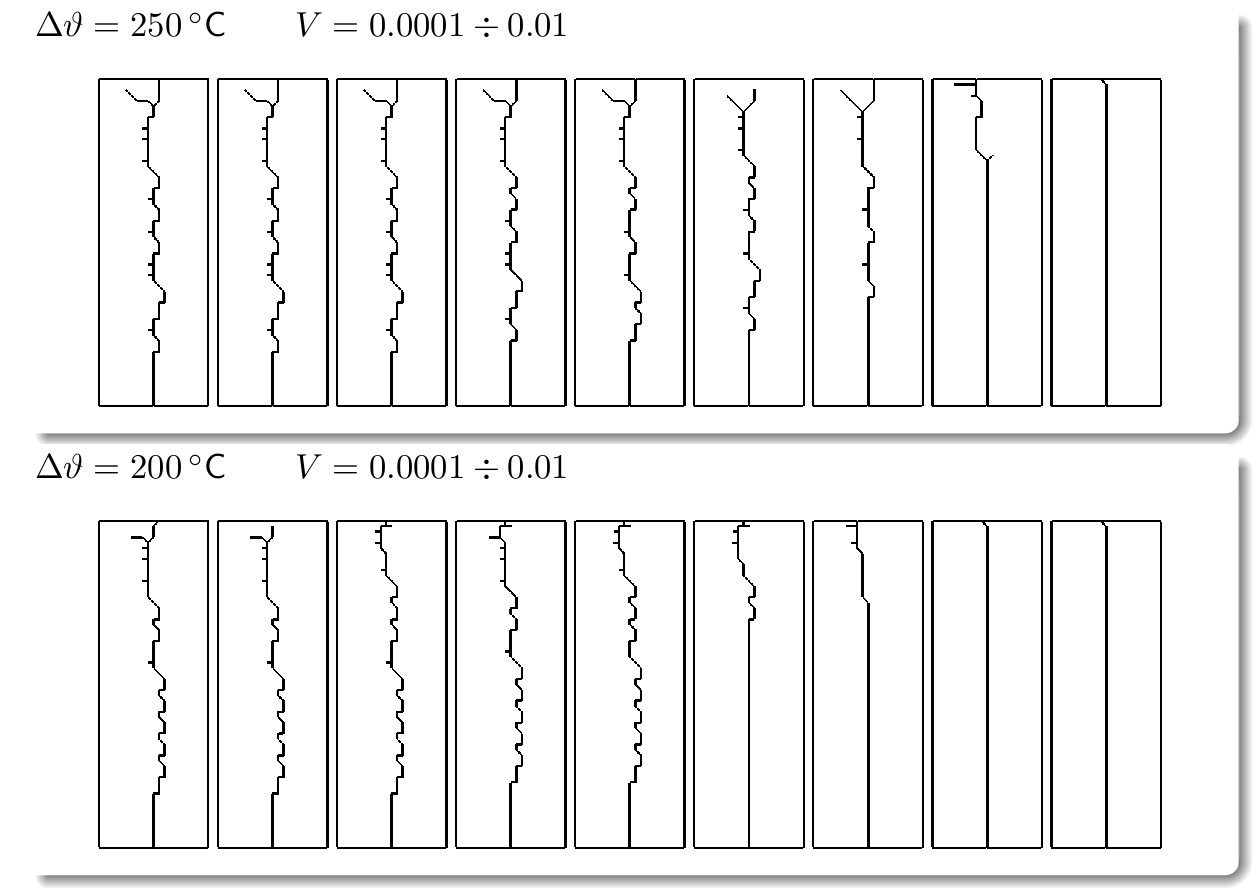
The direction of propagation of a crack and in particular the stability of straight propagation are fundamental problems of fracture mechanics not yet fully understood. One of the obstacles to this understanding is represented by the difficulty to perform well-controlled experiments. Multiple simple tests ([Yuse et al. 1993], [Ronsin et al. 1995]) have showed that a crack traveling in a thin glass plate with a thermal stress field undergoes a reproducible sequence of instabilities.
The work developed during my doctoral program aims to derive the instability of the straight crack propagation as an outcome of the numerical analysis by modeling fracture through a Dugdale-Barenblatt surface energy.
The thermo-mechanical problem is solved by a finite element code, in a two-dimensional approximation of the spatial set and imposing a quasi-static evolving temperature profile. The fracture propagation in brittle materials and in quasi-static conditions is modeled with cohesive finite elements, in order to predict the crack pattern depending on the stress concentration at the crack tip.

 (click on image to view the animation).
(click on image to view the animation).
The experiment is conducted by slowly pulling the sample from a hot region to a cold one, so that in effect a sharp thermal gradient moves across the plate. The plate is seeded with an initial notch.
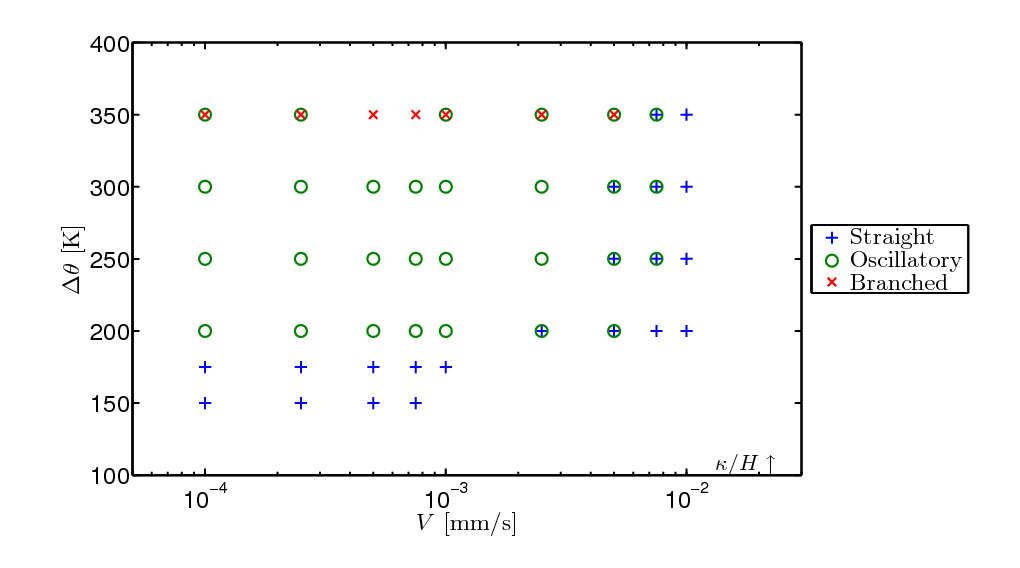
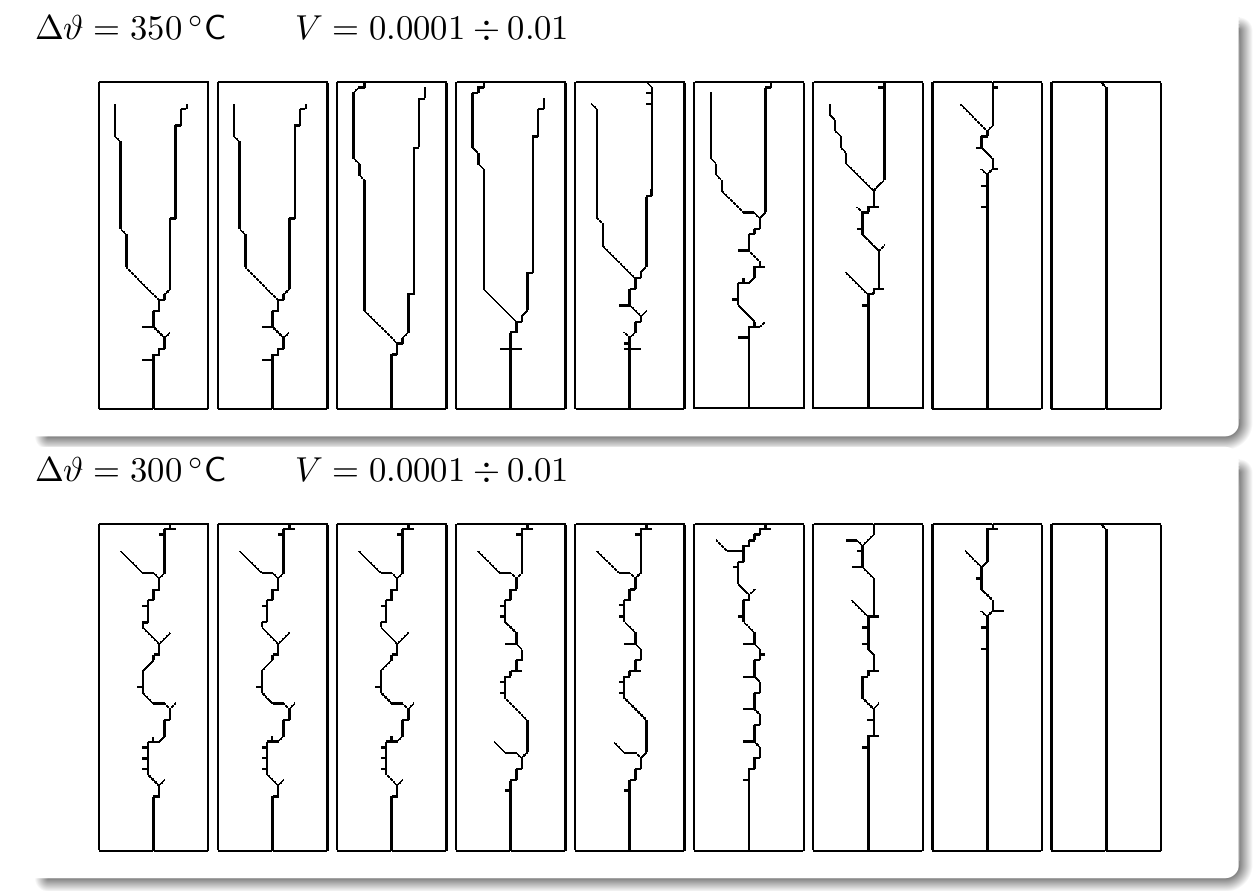
Fracture propagation in the glass plate due to sudden but controlled cooling shows drastic morphological changes depending on the experimental parameters. The patterns observed can be grouped in three categories:


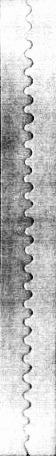
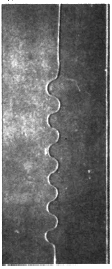

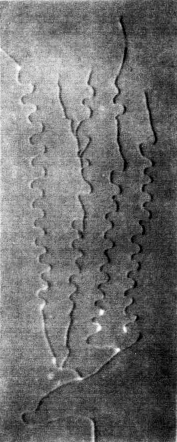 Experimental results [Yuse & Sano, 1995]
Experimental results [Yuse & Sano, 1995]
Classification of the fracture patterns obtained in the numerical texts by varying the temperature gap between hot and cold reservoir and the velocity of immersion of the plate into the cold bath.
|
Instability Problem
|