Eliza Genilo
*Eliza won the 2008 oral presentation contest. She presented her work at the annual American Society for Biochemistry and Molecular Biology meeting in the spring of 2009.
Dominican University of California
Keating Laboratory
Mentor: Sanjib Dutta
Summer 2008
Determining specificities and affinities between BH3 peptides and BcL-2 proteins using yeast surface display
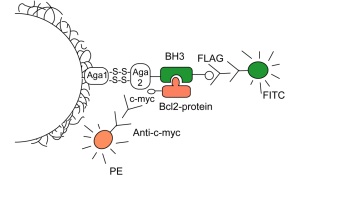
Apoptosis in every cell is determined by protein-protein interactions between the BcL-2 family, consisting of two subgroups: anti-apoptotic proteins (BcL-2, BcL-xL, BcL-W, McL-1, and A-1) and pro-apoptotic proteins. A specific subgroup of the pro-apoptotic proteins consist of "BH3 only" proteins. Our interest is to examine the specificities and affinities between the anti-apoptotic and the "BH3 only" proteins. These interactions occur between the amphiphilic regions of the BH3 peptides with the hydrophobic indentations of the BcL-2 proteins. A pool of BH3 peptides selected for specificity or promiscuity against BcL-xL or McL-1 were displayed on yeast cells and tested against several anti-apoptotic proteins. To understand specificities, the BH3 peptides were tested against these receptors using a high-throughput approach to determine which peptides were specific or promiscuous to the receptors. To understand affinity, a selected amount of specific BH3 clones were used for quantitative measurement of binding affinity. By observing specificities and versatilities in protein sequences, we can then manipulate the proteins to act as inhibitors or stimulators of favorable cellular outcomes.