Sirena Montalvo
University of Puerto Rico at Mayaguez
Drennan Laboratory
Mentor: Cintyu Wong
Summer 2008
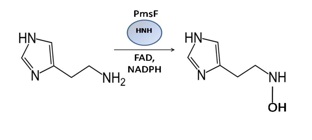
Crystallization of PmsF, a flavin-dependent hydroxylase
Pseudomonas entomophila is an entomopathogenic bacterium whose complete genome has been elucidated, yet there are gene clusters that are being studied to determine their biosynthetic pathways. One of the proteins in the biosynthesis of pseudomonine, a salicylic acid based siderophore, is PmsF. A siderophore is an important compound that helps iron intake in many soil microorganisms. PmsF protein is a flavin-dependent hydroxylase present in Pseudomonas entomophila and is responsible for the hydroxylation of Histamine in the biosynthesis of pseudomonine. The goal of this project is to find and optimize the conditions for crystallization of PmsF with its cofactor FAD. Crystal and precipitant screens were tested and optimized by varying pH and salt concentration. The first crystals grew in what seemed to be a single crystal with small rods growing from the sides. By the use of additives we were able to get crystals in a more diamond-like shape, but small. These crystallized with 0.1 M sodium acetate, pH 4.6 and 1.5-2.0M sodium formate. The final crystals were collected and used for x-ray crystallography data collection. The PmsF crystals are highly mosaic; and so further optimization must be done to get better quality crystals. When better crystallization conditions are found, PmsF Selenium-Methionine protein will be screened under the same conditions. Once crystallization of both proteins has been optimized and quality diffraction is obtained, the protein structure of PmsF can be solved.