Jenna Caldwell
Massachusetts Institute of Technology
O'Connor Lab
Mentor: Leslie-Ann Giddings
Summer 2009
Characterizing the Arylesterase Activity of the Human Protein c20orf3
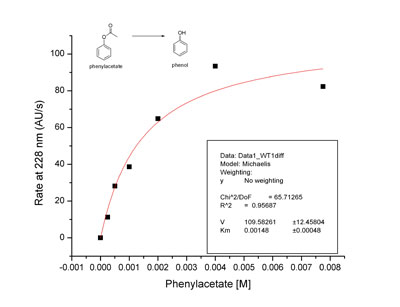
The protein encoded by the third open reading frame on human chromosome 20 (c20orf3) is homologous to two enzymes in the same superfamily: Catharanthus roseus strictosidine synthase (STS) and human serum paraoxonase (PON1). STS catalyzes a Pictet-Spengler condensation involved in the production of terpene indole alkaloids, but c20orf3 does not exhibit Pictet-Spenglerase activity. Structural homology models suggest that c20orf3 may be more closely related to the calcium-binding enzyme PON1. Perhaps c20orf3 shares PON1's arylesterase and organophosphatase activities. To test this hypothesis, c20orf3 was expressed in Saccharomyces cerevisiae, purified by Ni-NTA affinity chromatography, and assayed by high performance liquid chromatography for the hydrolysis of phenylacetate. Although preliminary data shows an enzymatic rate of hydrolysis that is less than threefold faster than the background rate, the data fit a Michaelis-Menten curve with K m = 1.5 mM, about three-fold higher than PON1. In calcium-chelating conditions, the enzymatic rate is significantly reduced relative to the background. Attempts to reconstitute the activity of EDTA-treated c20orf3 with Ca 2+ , Zn 2+ , Mg 2+ , and Mn 2+ were unsuccessful, suggesting that the metal ion may play a structural role in c20orf3 as it does in PON1. Residues corresponding to those known to be important for PON1 activity have been mutated; the mutants will be assayed to determine the importance of these residues for c20orf3 phenylacetate hydrolysis.