Fan Liu
Massachusetts Institute of Technology
Movassaghi Lab
Mentor: Omar Ahmad
Summer 2009
Synthesis and Reactivity of Densely Substituted and Electron-Rich Indoles
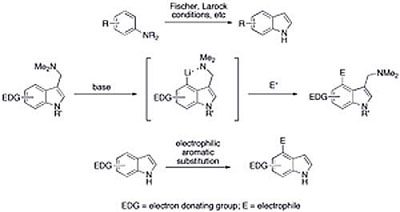
Indoles are prevalent heterocyclic compounds in nature and serve as important precursors for the synthesis of a wide variety of natural products, many of which show pronounced pharmacological effects and potential for clinical application. Our interest is to explore the reactivity of electron-rich indoles. Many methods of indole synthesis were explored, including the classical Fisher and Bartoliís indole synthesis, to evaluate the best synthetic strategy. Both electrophilic as well as nucleophilic methods were utilized for regioselective functionalization. The reactivity of the benzene substructure in indole was investigated via directed metalation and through further deactivating the pyrrole moiety. By exploring the properties of these electron-rich indoles, we can gain insight into their reactivity, which will prove invaluable in future natural product synthesis.