Alyson Weidmann
Columbia University
Stubbe Lab
Mentor: Joey Cotruvo
Summer 2009
Characterization of the Class Ib Ribonucleotide Reductase in Lactobacillus plantarum
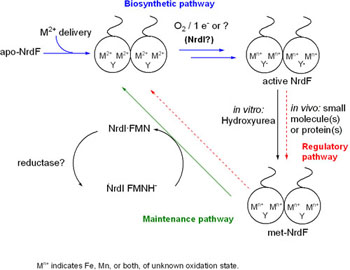
Ribonucleotide reductase (RNR) catalyzes the reduction of ribonucleotides to deoxyribonucleotides, a process essential to DNA biosynthesis and therefore indispensable to all organisms. Class Ia and Ib RNRs both consist of homodimeric subunits (?2 and ?2) - the ? subunit in each comprising the active site of ribonucleotide reduction, and the ? subunit containing a tyrosyl radical (Y·) that is necessary for RNR activity. In the class Ia ?2 subunit (NrdB), a diferrous center reacts with O2 to give the essential Y· in the form of a diferric-Y· cofactor, and it has been assumed that a similar mechanism occurs for the less well-characterized class Ib ?2 (NrdF). The formation and activity of diferric-Y· NrdF have been observed in in vitro studies; however, some studies have suggested that Mn may play a role in class Ib RNR activity in vivo. We have chosen to study Lactobacillus plantarum - which expresses class Ib RNR aerobically, does not require Fe for growth, and accumulates high levels of Mn - to study the assembly of the metallocofactor in class Ib RNR. We have cloned, expressed, and purified the L. plantarum class Ib ?2 (NrdE), NrdF, and NrdI (a flavodoxin with an essential but indeterminate role in the class Ib system), and characterized the system's metal binding, Y· formation, and activity in vitro.