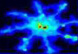

We are interested in the molecular basis of communication between brain cells, and the biochemical mechanisms underlying brain plasticity (such as learning and memory). Synapses are the specialized cell-cell junctions by which neurons of the brain communicate with each other. In response to environmental changes occurring throughout development and adult life, the brain reorganizes itself by adjusting the pattern and strength of its synaptic connections.
This plasticity of synapses occurs at both the biochemical and the morphological levels, allowing the brain to store information on short- and long-term time-scales. Our lab is interested in the dynamic molecular 'architecture' of brain synapses - the constituent proteins and the regulated network of protein-protein interactions that underlie the specialized structure/function of the synapse. Guided by such an architecture, we wish to understand the molecular signals that govern the formation of synapses during brain development, and the biochemical and cell biological mechanisms by which synapses change their strength and structure. The long-term aim is to correlate brain plasticity at the behavioral level with changes at the molecular/cellular level.
Our current focus is on
postsynaptic mechanisms, in which glutamate receptors play a primary role.
A variety of glutamate receptors are specifically clustered at postsynaptic
sites by their interactions with PDZ domain-containing scaffold proteins that
assemble large molecular complexes around each class of receptor. Components
of these complexes mediate the placement, trafficking and signal transduction
of glutamate receptors and play crucial roles in synaptic plasticity. The
molecular organization of the synapse and the functional roles played by specific
synaptic proteins are addressed by the techniques of molecular genetics, biochemistry,
cell biology and imaging, using in vitro, cell culture and in vivo systems
(primarily rodents). With an understanding of the cytoskeletal/signaling network
at the postsynaptic specialization, we are studying how this postsynaptic
architecture is dynamically regulated by phosphorylation and degradation during
synapse maturation and synaptic activity. Another area of interest is the
molecular control of synapse growth and dendritic spine morphogenesis. Our
studies are motivated by the expectation that some types of neurological and
psychiatric disease are caused by disordered molecular organization of brain
synapses


Menicon Professor of Neuroscience, Departments of Brain
and Cognitive Sciences and Biology, Associate Investigator Howard Hughes Medical
Institute, Investigator, RIKEN-MIT Neuroscience Research Center
Morgan H. Sheng
received his M.B.B.S. (MD) at Guys Hospital Medical School, London University.
He received his Ph.D. in Molecular Genetics from Harvard University. After
a postdoctoral fellowship at the University of California, San Francisco,
he joined the faculty of the Department of Neurobiology, Harvard Medical School
and the Massachusetts General Hospital. In 2001, he was appointed Professor
in the Departments of Brain and Cognitive Sciences and Biology, and joined
the Picower Center for Learning and Memory at MIT. He is a recipient of a
Lucille P. Markey Fellowship, and a Presidential Early Career Award. The Society
for Neuroscience has honored his achievements with the 1999 Young Investigator
Award.
 |