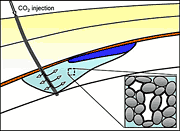
Storing carbon dioxide safely underground
A new analysis led by an MIT scientist describes a mechanism for injecting carbon dioxide (CO2) captured from power plants into briny porous rock deep underground, where it will be trapped naturally as tiny bubbles. The CO2 will slowly dissolve in the brine and over time adhere to the rock in the form of minerals such as iron and magnesium carbonates. Instead of escaping into the air, the captured CO2—a primary contributor to global warming—will be safely stored for centuries or even millennia.
Today, the world relies on fossil fuels to meet a significant fraction of its energy needs. But using fossil fuels—particularly coal—generates CO2 emissions. Continuing to rely on those abundant, inexpensive fuels while carbon-free energy options are developed will require capturing CO2 emissions from power plants and storing them underground, sequestered from the atmosphere.
Possible sequestration sites now being considered include geologic formations such as depleted oil and gas fields, unminable coal seams, and deep saline aquifers. However, a worrisome question arises: might the injected gas eventually leak back into the atmosphere through abandoned wells or underground cracks?
New research led by MIT Professor Ruben Juanes of civil and environmental engineering shows that such long-term leaking would not be an issue with sequestration in saline aquifers—porous rock formations bearing brackish water that are ubiquitous underground. CO2 captured during the lifetime of a power plant and injected into such a formation is unlikely to flow back up to the surface later.
“We have shown that this is a much safer way of disposing of CO2 than previously believed because a large portion—maybe all—of the CO2 will be trapped in small blobs in the briny aquifer,” said Juanes, who collaborated on the work with Elizabeth Spiteri of Chevron Energy Technology Co., Martin Blunt of Imperial College London, and Franklin Orr, Jr., of Stanford University. “Based on experiments and on the physics of flow and transport, we know that the flow of the CO2 is subject to a safety mechanism that will prevent it from rising up to the top just beneath the geologic cap,” he said.
The mechanism
The study shows that CO2 could be compressed as it leaves the power plant and injected through a well deep underground into a natural sublayer consisting of porous rock such as sandstone or limestone, saturated with saltwater. Because of its buoyancy, the injected gas will form a plume and begin to rise through the permeable rock.
Once the injection stops, the plume will continue to rise, but saltwater will close around the back of the gas plume. The saltwater and CO2 will juggle for position while flowing through the tiny pores in the rock. Because the rock's surface attracts water, the water will cling to the inner surface of the pores. These wet layers will swell, causing the pores to narrow and constrict the flow of CO2 until the once-continuous plume of gas breaks into small bubbles or blobs, which will remain trapped in the pore space.
“As it rises, the CO2 plume leaves a trail of immobile, disconnected blobs, which will remain trapped in the pore space of the rock until they slowly dissolve and, on an even larger timescale, react with rock minerals,” said Juanes. “It is a good example of how a process that occurs at the microscopic scale affects the overall pattern of the flow at the geologic scale.”
—Denise Brehm and Nancy Stauffer
Adapted from an article that appeared in On Balance, a publication of the MIT Department of Civil and Environmental Engineering.
This research was funded by industrial affiliates of the Petroleum Research Institute at Stanford.