Saying goodbye to batteries
Researchers at MIT are developing a new device that has the potential to hold as much energy as a conventional battery but could be recharged in seconds rather than hours, would last almost indefinitely, and won’t mind the cold. The device could prove the first economically viable alternative to today’s battery. It could one day yield a practical all-electric car and provide electricity storage critical to using intermittent energy sources such as solar and wind.
Just about everything that runs on batteries—cell phones, laptops, electric cars, missile-guidance systems—would be improved with a better energy-storage device. The battery continues to improve, but its basic concept hasn’t changed much since it was developed by Alessandro Volta in the 19th century.
Professor Joel E. Schindall of electrical engineering and computer science believes that what’s needed is a novel way of thinking. “I’m intrigued with the idea of using nanotechnology to transform ‘discarded’ technologies into the technology of choice,” he said. Now, using nanotube structures, he and his colleagues Professor John Kassakian and graduate student Riccardo Signorelli at MIT’s Laboratory for Electromagnetic and Electronic Systems are making a “synthetic battery” based on the ultracapacitor, an energy-storage device that’s been around since the 1960s and is used in many electronic devices to provide quick bursts of energy.
Conventional batteries store energy by using chemical reactions to trap ions that move from one electrode to the other. Batteries have a huge storage capacity, but—because of the chemistry involved—electricity can go in and out only so fast, and some is lost as heat.
In contrast, capacitors store energy in an electric field. The absence of chemical reactions has advantages. Capacitors can deliver energy quickly, and they can be charged up in minutes or even seconds. They can withstand temperature changes, shocks, and vibrations. And they can be recharged hundreds of thousands of times before they wear out. They’re thus much easier on the environment than today’s batteries, which must be tossed out after a few hundred charges.
But their capacity for storing energy is limited. The best version is the ultracapacitor. It contains an electrolyte, a fluid containing positive and negative ions; and its electrodes are coated with activated carbon, which is extremely porous and so provides a large surface area for storing the ions. Nevertheless, today’s commercial ultracapacitors store around 25 times less energy than a similarly sized lithium-ion battery can. As a result, they need to be much larger than batteries to hold the same charge.
Novel nanostructure
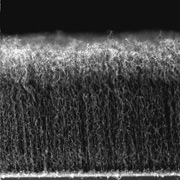
While ultracapacitors have many uses, they can’t compete with batteries when it comes to storing lots of electrical energy, noted Schindall. But a few years ago he read a journal article about vertically aligned nanotubes and began to wonder what would happen if he replaced the activated carbon with nanotubes. While the pores in activated carbon are irregular in size and shape, a nanotube “forest” might provide straight pathways so the ions could come in and out easily and pack together neatly—like sucking up paint with a paintbrush rather than a sponge.
Schindall and his colleagues have now developed a technique for growing nanotubes on an aluminum electrode. They put down droplets of a catalyst on the surface and pass a hydrocarbon gas over it at high temperature. The droplets grab carbon atoms out of the gas, and carbon nanotubes start growing upward, just like hair. Within ten minutes the surface is covered with millions of vertically aligned nanotubes, each one a thirty-thousandth the diameter of a human hair and 50,000 times as long as they are wide. By controlling the size and spacing of the droplets, they have made samples in which the nanotubes are just two ion-diameters apart—ideal for dense ion packing.
Detailed simulations suggest that their new device will work well. Indeed, the predicted energy-storage capacity is comparable to that of a lithium battery of equivalent dimensions—a similarity that they realized is no coincidence. The lattice structure of their device provides roughly the same storage space for ions as a battery does.
"When we were done, we realized that it wasn’t really a capacitor anymore," Schindall said. "Our adapted ultracapacitor actually mimics the molecular lattice of a battery but without the chemical reactions. It’s sort of a synthetic battery." The device could be made in all the sizes needed to replace today’s commercially available batteries—at roughly the same cost.
Schindall expects to have a working prototype finished in the next few months. If all goes well, the new nanotube-enhanced ultracapacitor could be on the market within five to ten years.