AXON GUIDANCE:
A second major goal of the lab is to understand how axons navigate to their targets to form the circuitry of the nervous system. During development, extracellular guidance cues are received and integrated by axonal growth cones which, in turn, coordinate the cytoskeletal remodeling required for migration to their targets. Growth cone filopodia, comprised of F-actin bundles, extend and sample the environment. The Ena/VASP protein family, comprised of Mena, VASP and EVL, is required for axon guidance and, in turn, regulates the assembly of F-actin networks to favor filopodia formation and elongation. Ena/VASP are targets of several signaling pathways involved in neuronal migration and axon guidance, yet it is still unclear how Ena/VASP function is linked to guidance receptors. Furthermore, Ena/VASP function is implicated in both attraction and repulsion, raising the interesting question of how molecules that promote actin polymerization and filopodia formation function during growth cone repulsion. Our global hypothesis is that Ena/VASP acts as a convergence point downstream of multiple guidance cues that regulates cytoskeletal responses.
Research


Understanding how a cell moves in response to environmental signals is a fundamental challenge in biology. We are interested in the machinery that links signaling pathways to cytoskeletal remodeling during guided cell movement. Our entry point into this problem is through the analysis of cytoskeletal regulatory proteins including the Ena/VASP family. We have shown that Mena, VASP and EVL, highly related proteins that comprise the Ena/VASP family, play pivotal roles in movement and shape change for a variety of cells, including fibroblasts, endothelial cells, epithelial cells, and neurons; Ena/VASP directly regulates actin filament network assembly, modulates morphology and behavior of membrane protrusions, and influences cell motility. There are three related Ena/VASP proteins in vertebrates: Mena, EVL and VASP. Our genetic studies reveal that Ena/VASP proteins play critical roles in neuritogenesis, axonal guidance and endothelial barrier formation and neural tube closure. Ena/VASP family members are concentrated at focal adhesion and in the leading edges and the tips of filopodia in migratory cells. Therefore, this protein family is perfectly positioned to serve a role in the early rearrangement of the actin cytoskeleton in response to migration cues.
CANCER:
One major goal of the lab is to study how Ena/VASP and associated signaling and cytoskeletal protein contribute to chemotactic responses in carcinoma cells. We hypothesize that Ena/VASP proteins integrate signals from second messenger pathways to execute required motile responses, and are thus not only well positioned to control chemotactic motility, but are in fact generally involved in systems that require guided motility
We are investigating the mechanisms underlying the changes in cell motility that arise during Epithelial-mesenchymal transition (EMT). EMT, a mechanism important for embryonic development, plays a critical role during malignant transformation. Normally, polarized epithelial cells connect to each other by forming adherens junctions, structures that are linked to the actin cytoskeleton by their intracellular components. Loss of apico-basolateral polarity and dissociation of junctions is accompanied by major remodeling of the actin cytoskeleton, leading to an elongated, migratory phenotype characteristic of mesenchymal cells. We have uncovered networks of coordinated changes in signaling and cytoskeletal regulation that are required for EMT and are examining how these changes may contributed to cancer cell invasion and metastasis.
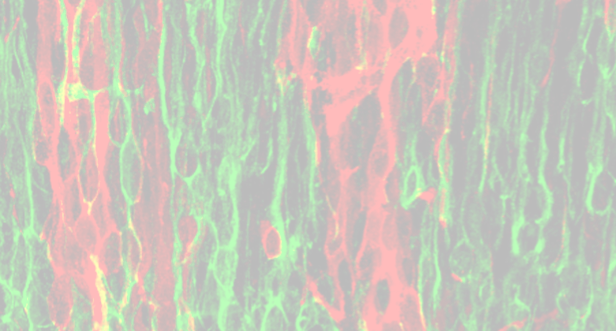
Mena (green), actin (red) in a fibroblast. Tuba (green), actin (red) in a melanoma cell.
