 |
      |
|
|
 |
Peter DeMuth Biological Engineering Grad Student
B.S. in Chemical Engineering (University of Maryland-2008)
B.S. in Biochemistry (University of Maryland-2008)
Hometown: Towson, Maryland |
Materials Development for
Transdermal Vaccination
1. Motivation
Vaccines currently represent a
significant strategy for the control of infectious disease on a global
level. However, despite the successes of modern vaccine development,
there remain several notable obstacles for the advancement of
vaccine-mediated improvements in global healthcare. Among these are
factors which limit vaccine availability, such as cost and the need for
cold storage, or vaccine efficacy and compliance, such as the ease and
speed of vaccine delivery. Many of the current limitations in vaccine
availability and administration are the result of obligate needle-based
delivery, which in addition to contributing to reduced speed, ease, and
compliance in administration, has been shown to contribute to reduced
overall safety due to needle re-use and needle-based injuries. The
inherent limitations of needle-based vaccination on global health,
together with emerging concern over global pandemic disease, has led to
a strong impetus to develop needle-free vaccination strategies which
have the potential to improve vaccine availability, enhance the ease,
speed, and safety of vaccine administration, and reduce
vaccination-associated costs world-wide. Thus, the development of
needle-free vaccination platforms has been identified by the World
Health Organization and the Centers for Disease Control and Prevention
as a major research priority in the improvement of global health.
2. Development of Polyelectrolyte Films
for Transdermal Vaccination
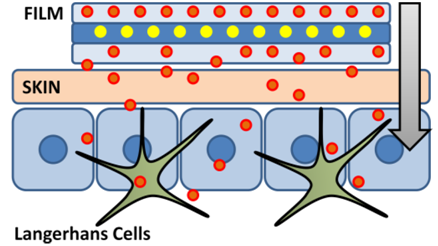
Vaccination through transcutaneous
delivery of antigen/adjuvant represents a promising strategy for
inducing protective immunity. For example, the abundance of Langerhans
cells, resident epidermal antigen-presenting-dendritic-cells, has been
shown to mediate both systemic and mucosal immunity through antigen
uptake, presentation, and subsequent activation of the adaptive immune
response. Therefore, transcutaneous delivery may produce a more robust
immune response relative to traditional intramuscular injection which
only elicits systemic immunity. In addition, transcutaneous delivery
platforms may provide opportunities for solid state vaccine
stabilization precluding the need for “cold chain” support, and
improving overall global availability. Unlike needle-based delivery,
transcutaneous delivery platforms may also allow for robust dosage
control for multiple antigen/adjuvant species and discrete time resolved
release allowing for more effective immune activation. This should
improve vaccine effectiveness and overall patient compliance. Finally,
transcutaneous vaccine delivery provides improvements associated with
the needle-free administration paradigm, including increased safety,
improved speed and ease of administration, and reduced training
requirements for health care providers.
The
central goal of my proposed work is the development of vaccination
platforms using polyelectrolyte films for the controlled encapsulation
and transcutaneous delivery of antigen/adjuvant combinations.
Specifically, I hope to develop strategies for film construction that
will 1) allow for the incorporation of a variety of antigen and adjuvant
species 2) provide for solid state vaccine stabilization resistant to
environmental fluctuation and 3) allow for defined and tunable
multi-component antigen and adjuvant release. To this end,
layer-by-layer (LbL) polyelectrolyte adsorption has been shown to be a
promising method for the construction of thin films with nanoscale
compositional control translating to fine temporal control of release.
LbL polyelectrolyte film construction is also well suited for
encapsulation of biologically active species due to mild aqueous
adsorption conditions. Variables defining film composition and
degradation offer a large experimental space for system optimization,
and additional control may be introduced through the use of films
incorporating hydrolytic polymer conjugates or macroscopic carriers such
as nanoparticles and micelles. Finally, the optimization of physical
substrates for film deposition such as micro-needles may further enhance
potential systems. I expect to explore this variable space to develop
robust platforms for transcutaneous vaccine delivery and characterize
these platforms for efficacy in producing protective immunity.
|
 |
