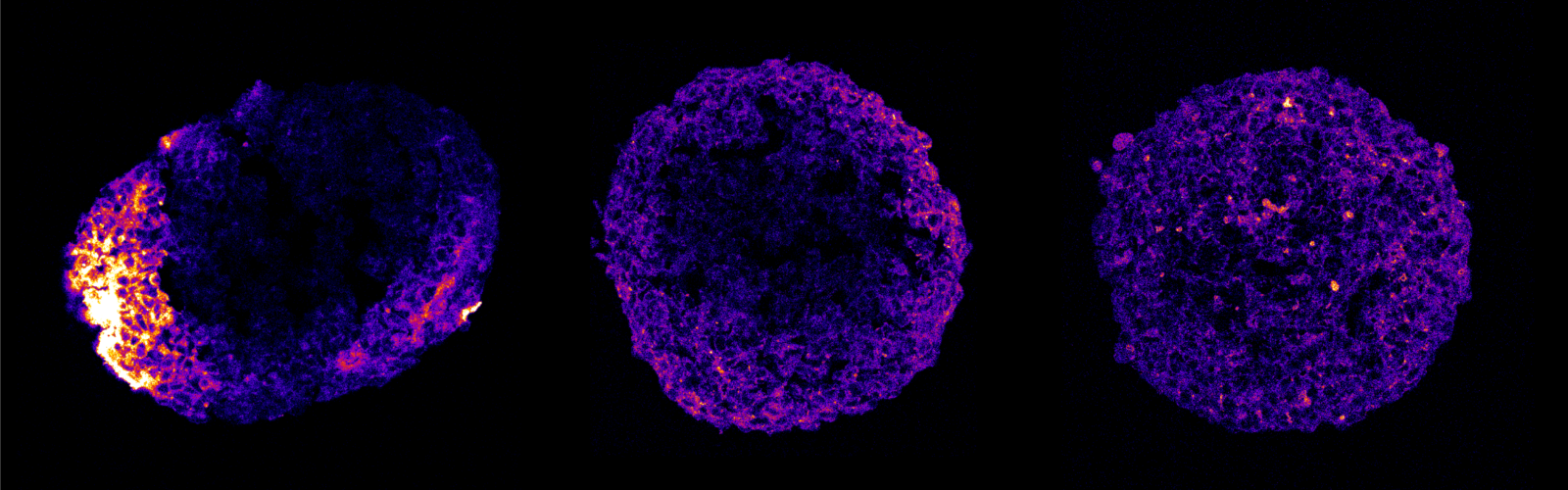
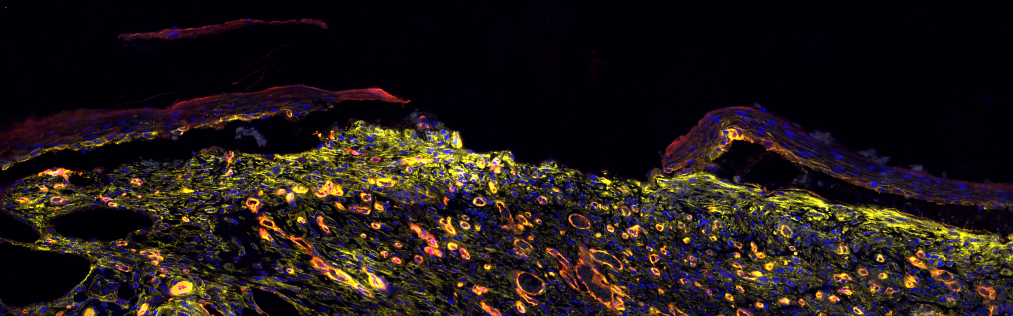
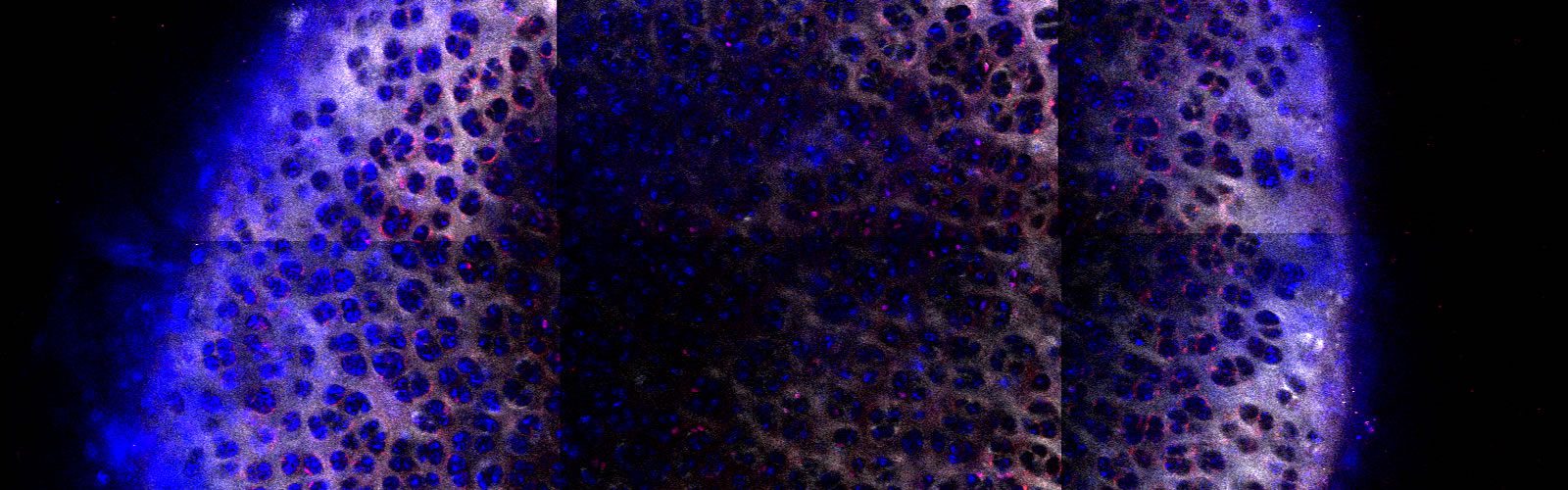
The Hammond Research Group at the MIT Koch Institute for Integrative Cancer Research focuses on the self-assembly of polymeric nanomaterials, with a major emphasis on the use of electrostatics and other complementary interactions to generate multifunctional materials with highly controlled architecture. The uniting theme of the lab – the understanding and use of secondary interactions to guide materials assembly at surfaces and in solution.