HST.175
09/05/00
1:30-4.00
Shiv Pillai
AN OVERVIEW OF THE IMMUNE SYSTEM
There
was a king reigned in the East:
There, when kings will sit to feast,
They get their fill before they think
With poisoned meat and poisoned drink.
He gathered all that springs to birth
From the many venomed earth;
First a little, thence to more,
He sampled all her killing store;
And easy, smiling, seasoned, sound,
Sat the king when healths went round.
They put arsenic in his meat
And stared aghast to see him eat;
They poured strychnine in his cup
And shook to see him drink it up:
They shook, they stared as white's their shirt.
Them it was their poison hurt.
-I tell the tale that I heard told.
Mithridates he died old.
From "Terence, this is stupid stuff"
By A.E. Housman
The survival of members of most vertebrate species to an age at which they can reproduce depends critically on their ability to resist infection. The specialized cells and organs that protect these animals from infection and death make up the immune system. This system continuously monitors every tissue and organ in the body for invaders. When the presence of cells or molecules that "do not belong" is confirmed, the immune system activates a battery of weapons designed to eliminate or contain the source of the infection. Growing awareness about inherited and acquired immunodeficiency diseases has helped underscore how absolutely crucial this protective system is for our survival.
Immunology is generally considered to be a daunting and complicated subject. As a result of the tremendous amount of information obtained over the last decade, this subject can now be recast in fairly simple terms. Indeed the study of immunology can provide an interesting and fairly simple introduction to many fundamental processes in biology. Obtaining an understanding, for example, of how the cells of the immune system develop from precursors, how these cells talk to one another, and how they respond to external signals, can provide important insights into the way all cells work.
The study of immunology, as distinguished from other areas of biology, revolves around two central questions. How does the immune system recognize the enormous variety of foreign shapes that must be targeted in order to ensure survival? How do we manage to discriminate between self structures and shapes that are foreign? The explosion in our knowledge of immunology at the molecular and cellular level has considerably simplified our understanding of the immune system. As a result, it is now possible to describe immunological phenomena such as the "generation of diversity" and "self-nonself recognition" in fairly simple cellular and molecular terms.
The primary goal of the immune system is to protect us from disease. One cannot seriously discuss this subject without reference to disorders that result either from the absence of an immune response or from an immune response gone awry. In this course we will briefly look at a number of diseases largely from a mechanistic point of view. Indeed there is little doubt that we are likelyto witness a new era in which an increased understanding of immune function will contribute to novel therapeutic approaches to immunodeficiency diseases, organ transplantation and cancer. Understanding the immune system may open the door to a very exciting and intriguing world.
Innate Immunity
The immune system as we know it in its highly evolved form is extraordinarily versatile. A considerably simpler version existed before the evolution of vertebrates. This more ancient immune system represents the principal defense machinery in invertebrates (animals such as insects and worms, which lack a backbone). The basic elements of this early protective system have been preserved in all animals including man. This primitive system, now frequently described as the "innate" immune system, is made up primarily of proteins called agglutinins and cells called phagocytes.
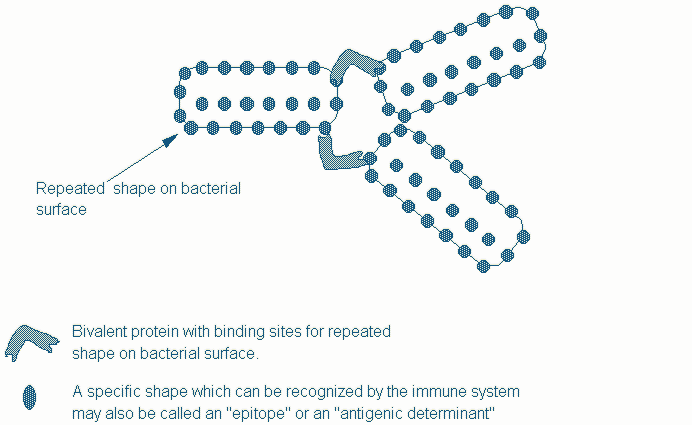
One of the underlying principles in nature's grand design for host defense is the requirement that the immune system should discriminate between self and "non-self". Agglutinins are multivalent proteins which recognize structures that are not found on host cells but which are commonly found on the surface of microorganisms. The term multivalent here refers to the presence of more than one recognition site in a single protein. Having more than one recognition site allows agglutinins to "agglutinate" or clump target cells such as bacteria with the appropriate coat sugar for instance (Figure One). An agglutinin which recognizes carbohydrates is called a lectin. Lectins that are capable of specifically agglutinating certain microbes are secreted by cells of the host. These lectins do not recognize sugars on the surface of host cells and thereby discriminate between self and non-self. Phagocytes (phagos=to eat) are specialized cells which engulf invading microorganisms. They either use specific receptors which discriminate between self and foreign shapes or they recognize microbes that have been coated by host agglutinins.

Figure One. Agglutinins must have more than one binding site to enable them to clump or agglutinate their targets.
Another important component of the innate immune system is a set of circulating complement proteins. These proteins obtain their name from their ability to complement the function of antibody molecules in the immune system. They help destroy target cells coated by antibody proteins by punching holes in the cell membrane. The concentration of sodium chloride outside cells is relatively high - the holes in the membrane permit salt and water to rush into targets cells which swell up and burst, a process kmown as lysis.. Complement proteins however existed in invertebrates before the evolution of antibodies. A subset of complement proteins can directly coat certain bacteria, thus either lysing them or targeting them for ingestion by phagocytes. The complement system will be discussed in a subsequent lecture.
Vertebrate immune systems recognize a tremendous diversity of shapes
The innate immune system has remained valuable over millions of years of evolution. We, as a species, retain this machinery as a first line of defense even though we possess a superimposed and more refined system for host protection. This more sophisticated and specific immune system evolved in vertebrates probably because the innate immune system could not effectively guarantee the survival of creatures with a longer potential life span.
The earliest vertebrates were very primitive fish and it is in these animals that the evolution of the immune system made a quantum leap. The immune system in its highly evolved form permits the specific recognition of an incredible variety of shapes that are identified as being foreign. Acquired or specific immunity as seen in vertebrates, is designed to be able to deal with any conceivable foreign shape that nature can present or that any synthetic chemist may choose to create at any time in the future. A particularly interesting characteristic of vertebrate immune systems is their ability to respond more readily to foreign shapes that they have seen before, a phenomenon known as immunological memory.
Constructing models for the immune system
The immune system in vertebrates continuously conducts "search and destroy" missions looking for shapes that are foreign. In order to be effective it must recognize an immense number of foreign structures. It is critical that the shapes being recognized be distinguished from self structures. Each recognition event results in an extremely aggressive, xenophobic response aimed at the destruction of any cell that might bear a foreign shape. The immune system, in addititon to seeing and responding to an enormous number of foreign shapes in a specific manner, also has the ability to respond more efficiently to a foreign structure that it has seen before, apparently "remembering" that it was previously exposed.
Given these three important characteristics of the immune system - a wide repertoire, the ability to discriminate between self and non-self, and the phenomenon of memory, can one actually construct a simple model to explain how this system works? One requirement to be met by any model would be for the synthesis of many different molecules that recognize foreign shapes. These "immune receptor" molecules should obviously either be on cell surfaces or secreted in order for them to be able to see novel shapes on invaders. Indeed, all of the the above broad characteristics of the immune system were inferred in the early decades of this century only after the discovery of proteins in the blood called antibodies. Antibodies were discovered by Kitasato and von Behring in 1890 as substances in the blood produced in response to injected toxins. These antitoxins, later called antibodies, were capable of neutralizing toxins and protecting individuals from disease. Ehrlich recognized that antibodies must only be directed against non-self shapes. Studies on antibody formation in rabbits revealed that a second exposure to a foreign substance resulted in a more rapid and vigorous appearance of antibodies, suggesting the existence of memory. This knowledge led to the development of two broad categories of models describing how the immune system is structured. It is an interesting exercise to try and construct these kinds of models for ourselves.
It is assumed that perhaps 10 million different antibodies can be made by the immune system. Does every immune cell have the capacity to make and secrete all the 10 million or more different antibody species? In a simple version of one type of model we might assume that all immune cells make every possible "immune receptor" and secrete all of them at all times. It could be postulated that exposure to a foreign shape may drive exposed cells towards the expression and secretion of a single type of "immune receptor". In such a model each immune cell would recognize a specific shape through one of the antibody species it makes, and then this recognition event would somehow induce this cell to commit itself to making just this single specific type of antibody. The above scheme of events represents an example of an "instructive" model for the immune system. A specific shape is recognized by an immune cell; this recognition event induces this cell to be subsequently committed to the synthesis of a single antibody (Figure Two).
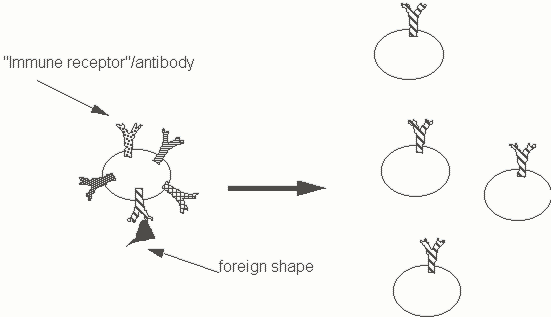
Figure Two. An instructive model for the immune system.
No evidence has actually emerged to support any instructive model for the immune system. In retrospect we recognize that the kind of model depicted in Figure Two is extremely unlikely for a number of reasons. If a single cell actually synthesized over a million different proteins, each protein would likely be made in minuscule amounts. Moreover, since most vertebrate cells express no more than 50, 000 to 100, 000 different genes, it is extremely improbable that a single immune cell could ever express 10 million different receptor genes. Indeed we just don't have enough functional DNA to code for 10 million different genes - the immune system has solved this problem in a clever way that we will discuss in detail . Although instructive models have long been abandoned they do allow us to begin to think about the cellular organization of the immune sytem and the phenomenon of cell selection. It is useful to think in terms of immune receptors as molecules that are expressed on the surfaces of immune cells. Encountering cognate foreign shapes (shapes that fit into immune receptors in a lock and key fashion) may generate signals that cause these cells to be selected resulting in their proliferation and activation.
An alternative model for the immune system suggests that individual immune cells each express only a single receptor that is distinct from receptors on other immune cells. Each immune cell is therefore unique from the moment of its inception and can recognize only one shape. In such a model, cells bearing receptors that recognize self structures are somehow inactivated, thus ensuring self-non self discrimination. Other immune cells that do not express receptors against self structures make up the immune repertoire. This repertoire is made up of cells with receptors that are specific for non-self or foreign structures. This model, as we shall see below, represents as simple a picture of the vertebrate immune system that can be accurately constructed today.
Lymphocytes and the clonal selection hypothesis
A major strategy in the evolution of vertebrate immune systems was the development of a means to synthesize a tremendous repertoire of distinct molecules that can recognize and thus help dispose of an almost infinite variety of pathogens. The cells responsible for the vast repertoire of the immune system, so far referred to as immune cells, are known as lymphocytes. One lineage of lymphocytes develops in the bone marrow of most vertebrates and in a specialized organ in birds known as the bursa of Fabricius. These lymphocytes are known as B lymphocytes (for bursal or bone-marrow derived).
Each individual B lymphocyte makes a distinct protein known as an antibody molecule which can recognize a different shape from those recognized by other antibodies made by other B cells. An antigen is most simply defined as any structure that can be recognized by the immune system. A given antigen may trigger the expansion of a specific B cell expressing the appropriate antibody molecule on the cell surface. This type of distinct antibody molecule expressed on the cell surface is known as an antigen receptor or a membrane immunoglobulin. Each antigen receptor bearing cell may expand on exposure to a cognate antigen to form a clone of B lymphocytes that secrete a specific antibody molecule (Figure Three). The concept of distinct antibodies being synthesized by individual lymphocytes which can be clonally expanded by a specific cognate antigen was proposed by Burnet and Talmage in 1955. The clonal selection theory is a central paradigm of vertebrate immunology.
While it is useful to think of the immune system as an army of lymphocytes primed to recognize and destroy invaders, we now appreciate that this is not a faceless army, but a force made up of highly specialized individual soldiers. When an invader enters the body, the majority of lymphocytes it encounters may be totally impervious toits existence. The few specialized lymphocytes with the "right" receptors that specifically recognize antigenic shapes on the invader are stimulated. They respond by proliferating (clonal expansion) and also activate an impressive battery of weapons designed to wipe out this pathogen.
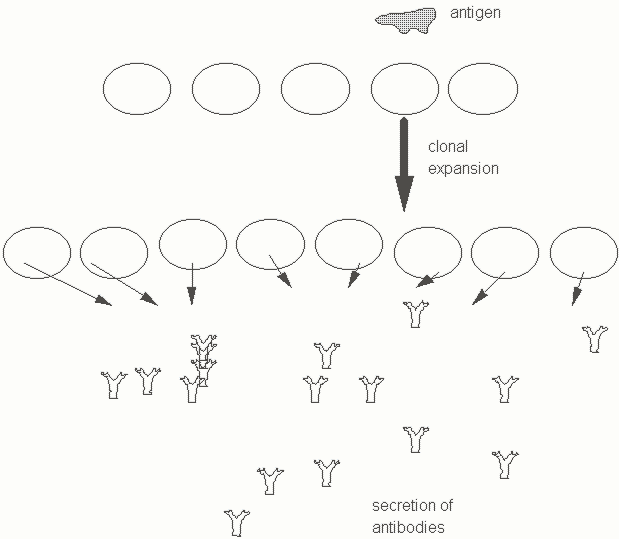
Figure Three. A representation of the clonal selection hypothesis. Individual lymphocytes express distinct surface receptors for antigen. A specific antigen binds to a B cell clone and this leads to the expansion of this clone and its differentiation into an a specialized form of antibody secreting B lymphocyte.
A simple overview of the structure and function of antibodies
Antibodies can deal with a vast variety of pathogens. Indeed the antibody repertoire is immense - the binding or combining sites of antibodies may be able to recognize somewhere in the range of 10 million different shapes. Antibodies share with agglutinins all the features that contribute to the elimination of pathogens, and can also contribute to host defense in some ways that the innate immune system cannot.
Antibodies are also called immunoglobulins. Proteins in the non-cellular portion of blood that has been prevented from clotting (the plasma) or from the straw-coloured liquid portion of clotted blood (the serum; this lacks some clotting proteins found in plasma) can be crudely fractionated according to charge, by a process known as electrophoresis. The slow moving g-globulin fraction contains antibodies, hence the name immunoglobulins. The existence of antibody secreting tumors known an myelomas or plasmacytomas greatly facilitated the study of immunoglobulins and their structure. Tumors are in general derived from a single cell. A single cell and its progeny are generally referred to as a clone and tumors are therefore clonal (or monoclonal) outgrowths. Myelomas and plasmacytomas are derived from differentiated antibody secreting B lymphocytes known as plasma cells These plasma cell tumors each produce large amounts of a single or monoclonal antibody.
From our knowledge of the biology of agglutinins that mediate innate immunity, we might make certain predictions about antibody structure and function. Antibody molecules secreted by individual B lymphocytes would, of course, be predicted to differ in their combining sites for antigen. It might perhaps be logically assumed that all antibody molecules share common features that enable them to agglutinate particulate antigens, to assist in the phagocytosis of foreign structures that they coat, and to bind to complement proteins. Indeed, antibodies possess all these predicted features. The basic structural unit of all antibodies is, as shown in Figure Four, a tetramer (a protein made up of four polypeptide subunits) of two heavy and two light chains, containing two identical binding sites for antigen. This bivalent structural unit is Y shaped with the two binding sites set sufficiently apart to facilitate agglutination or clumping of target microbes. The portions of the heavy chain and light chain that are involved in antigen recognition are at the N-terminal ends and are referred to as V (or variable) domains. The remaining portions of the heavy and light chains are referred to as constant (or C) regions. Each light chain constant region contains a single constant domain (CL). A domain refers to a portion of a protein which can be separated from the rest of the molecule and still fold into its correct shape. The domains in immunoglobulin molecules have a typical three dimensional structure which is now referred to as an immunoglobulin domain and is found in a variety of proteins (many of which existed before the evolution of immunoglobulins). Each heavy chain constant region is made up of three or four domains (CH). The hinge region lies between the CH1 and CH2 domains of the IgG heavy chain and confers flexibility to the molecule, allowing it to "bend" more widely, thus making it a better agglutinin.
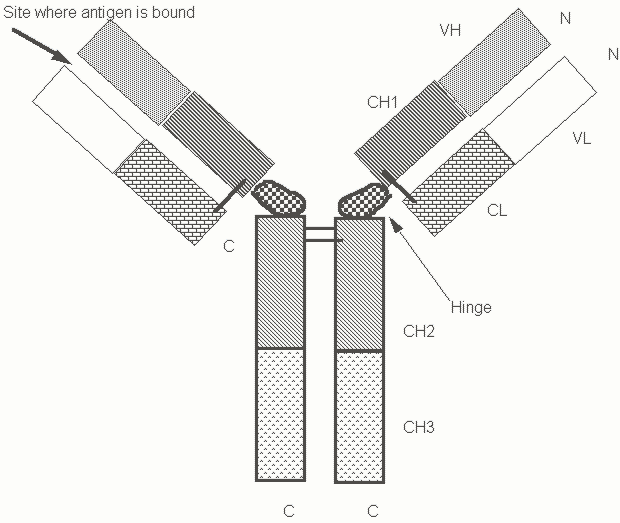
Figure Four. A schematic representation of an IgG antibody. N refers to the amino-terminus and C refers to the carboxy-terminus of each of the two polypeptide chains.
Humoral and cellular immunity
Immunity mediated by circulating antibodies or agglutinins is called humoral immunity - the term "humor" refers to body fluids. Antibodies are secreted by activated clones of B lymphocytes. Serum that contains antibodies against an extracellular pathogen may be transferred to another recipient, conferring passive resistance or passive immunity . This is in contrast to the immunity conferred by an actual infection or immunization event which is referred to as active immunity.
A second arm of the immune system is referred to as cellular or cell mediated immunity. Cellular immunity is mediated by thymus derived T lymphocytes. Just as distinct membrane antibody molecules constitute distinct receptors for antigen on individual B lymphocytes, individual T cell clones possess distinct T cell antigen receptor molecules each of which recognizes a different antigenic shape.
T cells see only see antigens bound to cell surfaces
Why do we need T cells? What do T cell receptors see that cannot be seen by antibodies? Humoral immunity is directed against extracellular microbes and antibodies can also block viruses and other pathogens from entering host cells. However antibodies cannot enter or "look into" cells. Viruses and other intracellular pathogens which are established within host cells therefore cannot be seen by antibodies. T cell receptors however are designed to, in a sense, look inside target cells. These receptors monitor whether certain specialized cell surface molecules are bound to fragments of proteins known as peptides that are generated within cells. Peptide fragments are bound to a specialized cleft in molecules known as MHC proteins (the reason why these proteins are called MHC molecules will be discussed later). These MHC proteins carry peptides to the cell surface. If the peptides "presented" on the cell surface are derived from foreign or non-self proteins then they will be recognized by specific T cell receptor bearing T cells. T cell receptors are essentially designed to ignore free antigens and to only recognize peptide complexed to MHC molecules; they thus specifically recognize cells that have been apparently taken over by intracellular pathogens. As a result T cells can recognize and target infected cells that would be left untouched by antibodies (Figure Five).
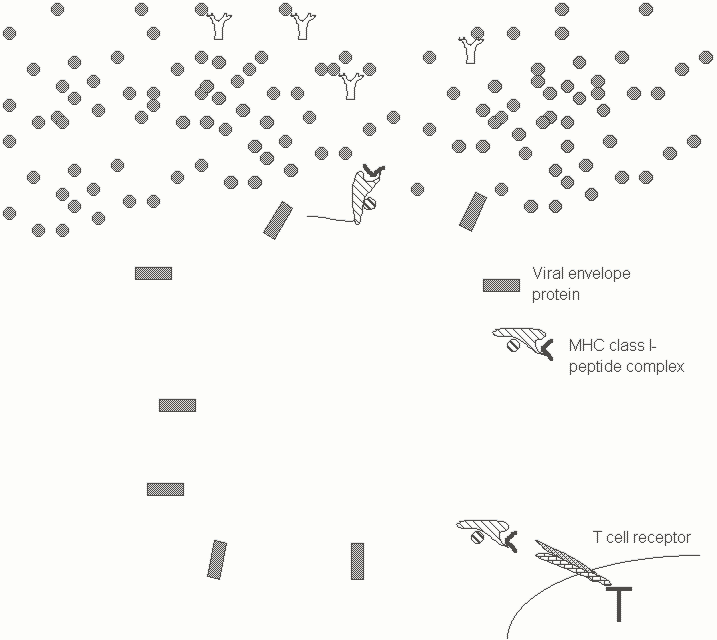
Figure 5. Antibodies can recognize viruses but sometimes fail to "reach" virus infected cells. T cell receptors ignore free antigens and focus on cell surfaces. (The MHC molecules depicted here are known as MHC class I molecules).
B and T cell development both involve repertoire generation and the deletion or silencing of self reactive cells
Two unique goals need to be achieved during lymphocyte development. The first is the generation of a diverse immune repertoire - it is necessary to have lymphocytes with receptors that can recognize a very wide variety of shapes. The second requirement is that lymphocyte receptors should be directed against foreign shapes and not against structures prevalent in the host. These requirements are generally referred to as the "generation of diversity" and "self-nonself discrimination".
The generation of diversity will be discussed in some detail in subsequent lectures. Immunoglobulin and T cell receptor genes come in pieces. The V domain of each antibody light chain for instance is encoded by one of a large number of V gene segments. In individual B cells one particular V gene may be selected and physically joined to DNA that encodes the constant regions of the light chain. This recombinational process by which different variable gene segments are joined to DNA encoding constant regions is termed DNA rearrangement. The use of this rearrangement process permits, as we shall see, a relatively small amount of the genome to be shuffled around to generate a large number of immune receptors
Once lymphocytes with a wide repertoire have been generated, the need exists to ensure that this repertoire is directed only against foreign antigens. The primary means by which self-nonself discrimination is achieved is by eliminating cells that make receptors directed against self antigens. When lymphocytes are generated in central lymphoid organs (the bone marrow for B cells and the thymus for T cells) they pass through a 'developmental window' during which time they respond to antigen not by proliferating but by being inactivated. Very tight binding to antigen during this time period leads to cell death. As lymphocytes pass through this developmental window the antigens that they usually encounter are self antigens. Those lymphocytes with receptors that bind very well to self antigens are thus eliminated. The process by which cells expressing self reactive receptors are eliminated is known as negative selection or clonal deletion or central tolerance. Following development in central lymphoid organs lymphocytes acquire the ability to leave these organs and to seed lymph nodes in the periphery (Figure Six).
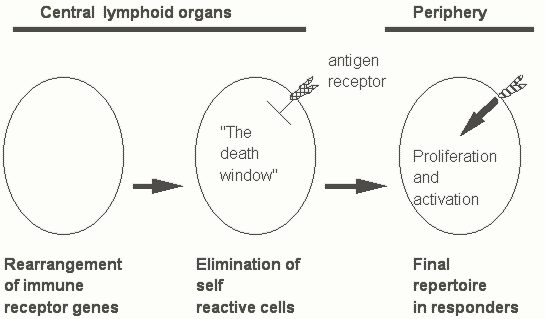
Figure Six. A simple overview of lymphocyte ontogeny.
In summary, the immune system in vertebrates has two arms. Humoral immunity is the province of B cells and cell mediated immunity is generated by T lymphocytes. Individual lymphocytes have distinct antigen receptors that each recognize a specific shape. The tremendous diversity of antigen receptors is primarily achieved by gene rearrangement. During lymphocyte development most self reactive lymphocytes are tolerized by being triggered by self antigens during a narrow developmental window when cells are wired to be turned off. Beyond this window, cells respond to foreign antigens and divide and differentiate into effector cells of the immune system. Once activated by antigens the immune system exhibits a form of memory and subsequent antigenic challenges meet more aggressive responses.
Recommended reading
Abbas et al. 3rd ed. Chapters 1 and 2; Janeway and Travers 5th ed. Chapter 1.
Additonal reading:
Panum, P.L. (1847) Beobachtungen uber das Maserncontagium. Virchows Arch. 1, 497-502
The epidemiological argument for immunological memory was made before anyone had actually described the immune system.
GETTING YOUR BEARINGS
This first lecture is meant to be an introduction. Many of the concepts will be reinforced in Lecture II. The terms listed in the glossary above should begin to make sense. In particular you should begin to get a feel for the differences between innate/natural immunity and adaptive/specific immunity
Glossary for Lecture 1:
Active immunity: Immunity conferred by immunization (as opposed to Passive Immunity)
Adaptive Immunity: aka Specific Immunity. Lymphocyte dependent immune systemwhich has very diverse antigen receptors and exhibits memory.
Agglutinins: Multivalent proteins that recognize structures on cell surfaces or on other proteins. They can precipitate pathogens or coat them for removal by phagocytes. They may "fix" complement proteins on the surfaces of pathogens.
Antibodies: Y shaped proteins of adaptive immunity, which are the bullets of the humoral immune system (B lymphocytes are the warriors).
Antigens: Structures recognized by the immune system. May or may not be an immunogen.
B lymphocytes: "Bursal" or "Bone-marrow" derived lymphocytes. Responsible for antibody mediated humoral immunity.
Cell Mediated Immunity: Immune responses mediated by cells.
Complement: Serum protein cascade which is generally activated either by certain pathogens or by agglutinin or antibody coated microbes.
Humoral immunity: Immune responses mediated by circulating secreted molecules
Immunogens: Antigens that can induce specific immune responses. Some antigens can be recognized by the immune system but do not induce responses — they are NOT immunogens.
Innate Immunity: aka Natural Immunity. Immune system which responds in minutes and hours to noxious stimuli. Less specific than Adaptive immune system and does not exhibit memory.
Natural Immunity: aka Innate Immunity
Passive Immunity: Immunity that is passively obtained without immunization by the transfer of cells or serum
Phagocytes: Cells which gobble up microbes and other particles
Specific Immunity: aka Adaptive Immunity
T lymphocytes: Thymus derived lymphocytes. Mediate cellular immunity during adaptive immune responses.