
HST 175
September 19th, 2000,
1.30 to 4 P.M.
Shiv Pillai
B LYMPHOCYTE DEVELOPMENT AND ACTIVATION
Recommended Reading: Abbas et al., 4th edition, Chapters 7 and 9; Janeway et al., 4th edition, Chapters 6 and 9
B cell development is initiated in the fetal liver prior to birth and continues in the bone-marrow subsequently. The B-1 lineage develops primarily from fetal liver derived stem cells. Conventional B cells are derived from bone-marrow derived HSCs and are sometimes referred to as B-2 cells.
While the initial steps of B lymphoid commitment and development depend upon signals from stromal cells, unique B lineage specific selection events drive the developmental program after immunoglobulin gene rearrangement is initiated. Two of these events are based on the nature of the rearrangements that have occurred at the heavy chain locus. Cells in which a particular D segment reading frame can be used following D to JH rearrangement are selected against, although this phenomenon is poorly understood, and may be restricted to the mouse. Approximately one-third of developing pre-B cells in the bone-marrow make in-frame V to DJH rearrangements and these cells are positively selected (positive selection I). Selection events at the pre-B stage in the bone marrow are probably distinct from those in the fetal liver as will be discussed below. In the bone-marrow pre-B receptor signaling mediates both positive selection I as well as allelic exclusion at the IgH locus. Once both heavy and light chain genes are rearranged and expressed, immature B cells may also be positively selected in response to antigen receptor mediated signals (positive selection II). If these cells express self-reactive antigen receptors, they may be subject to receptor editing) or they may be selected against by means of a deletional process. An overview of the different stages of B cell development, following the nomenclature developed by Hardy and colleagues, is provided in Figure 1.

Figure 1 A simplified overview of B cell development. The nomenclature is largely that of Hardy and colleagues. Stage A includes uncommitted progenitors as well as the earliest cells in the bone-marrow that are irreversibly committed to the B lineage but which have yet to rearrange their Ig heavy chain genes. D to JH rearrangement peaks during stage B. V to DJ rearrangement is initiated during stage C. Cells that make productive rearrangements receive signals via the pre-B receptor and expand into a population of proliferating cells represented by stage C'. This transition depends in man, (but not in the mouse), on signals delivered by Btk (defective in X-linked agammaglobulinemia or XLA in humans).
Stromal factors and the development of B cell progenitors from hematopoietic stem cells
HSCs presumably respond to external stimuli that commit them to distinct differentiation programs. These external stimuli presumably initiate signaling pathways which induce specific nuclear factors to drive the process of differentiation. A challenge in the field is to connect specific ligands, receptors, and signaling pathways, to transcription factors that drive the developmental process. A number of extracellular factors may contribute to the process of B cell commitment and some of these are considered in Figure 2.

Figure 2. Some of the factors that contribute to B lineage commitment and differentiation. cell surface receptors that are critical include the Flk2/Flt3 receptor tyrosine kinase, the a4b1 integrin, the IL-7 receptor and the CXCR4 chemokine receptor. The Pax-5 transcription might be induced downstream of IL-7R and the Ras/ERK pathway might contribute to the activity of Ets family transcription factors that drive development.
Pro-B and pre-B receptor signaling
A rearranged immunoglobulin heavy chain gene encodes two forms of heavy chain protein, a membrane form and a secreted form. mRNAs specific for these two forms are generated as a result of differential splicing and polyadenylation. It is the membrane form of the immunoglobulin heavy chain that is involved in signal transduction, as part of the pro-B receptor in pro-B cells, the pre-B receptor in pre-B cells, and the antigen receptor in B cells. Broad differences in membrane and secretory immunoglobulin heavy chains are highlighted in Figure 3
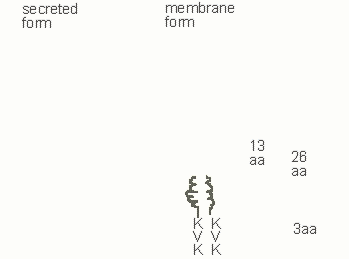
Figure 3.Membrane IgM heavy chains possess a 13 amino acid (aa) negatively charged extracellular connecting piece, a 26 amino acid membrane anchor and a short cytoplasmic tail (KVK)
The membrane and secretory forms of immunoglobulin heavy chains of all immunoglobulin isotypes are generated by alternative processsing. In Figure 4 the selective polyadenylation and alternative splicing events involved in generating these two messages are highlighted. Through most stages of B cell differentiation, RNA processing generates both forms of heavy chain message. However in plasma cells the secretory form is more efficiently generated.
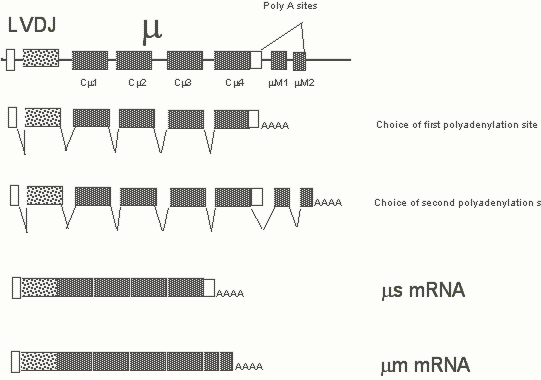
Figure 4. Membrane and secretory forms of the m heavy chain are generated by alternative splicing and selective polyadenylation. The relative levels of one general polyadenyaltion factor Cst 64 determines the ratios of the membrane and secretory forms that are generated.
Cell selection and allelic exclusion mediated by the pre-B receptor
Pre-B cells which have made in-frame rearrangements at the heavy chain locusafter V to DJH rearrangement and which can therefore synthesize a m protein are positively selected. This selection event depnds on signals from the pre-B receptor. The pre-B receptor mediated survival signal is dependent on the presence of the membrane immunoglobulin heavy chain, surrogate light chains, the Iga/b heterodimer, Syk and (in man, but not critically so in mice) Btk. This latter kinase is defective in X-linked agammaglobulinemia, an inherited disease in which the pre-B to B cell transition is blocked. Formal evidence suggesting that Src family kinases are involved in the generation of the pre-B receptor mediated survival signal is lacking, presumably because of the large number of Src family kinases that can associate with the pre-B and B cell receptors. However since both Syk and Btk are activated by Src-family kinases, it is extremely likely that Src family kinases associated with the pre-B receptor play a role in generating a signal for survival and allelic exclusion. The pre-B receptor is largely intracellular although it can be detected with some difficulty on the surface of pre-B cells. No ligand has been identified for this receptor and it is likely that it signals constitutively, being triggered by the act of pre-antigen receptor assembly.
It is formally possible that a yet to be identified ligand exists for the pre-B receptor. However truncated immunoglobulin heavy chains lacking ligand binding regions can participate in the generation of pre-B survival and progression signals. Given the fact that pre-antigen receptors are generated, not to respond (like most receptors) to external clues, but to monitor if the "right" kind of rearrangement has taken place within a given cell, it is not unresonable to suspect that such a receptor does not require an external ligand for signal initiation. The fully assembled receptor, because of some structural feature of the surrogate light chains, may be constitutively "on" or may respond to a ligand made by the pre-B cell itself.
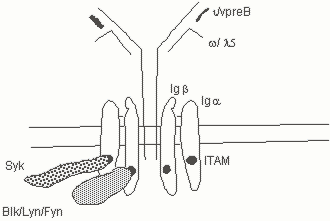
Fig. 5. The pre-B receptor is made up of the membrane form of the heavy chain, surrogate light chains, and the Iga/b heterodimer. Src family kinases and Syk are associated with the receptor.
Allelic exclusion may be achieved bycompletely different mechanisms at heavy and light chain loci. Alleleic exclusion at the heavy chain locus appears to be a regulated process dependent on a feedback signaling mechanism, while at at least one light chain locus (k) this process might occur by the intrinsic restrictionof accessibility to a single chromosome in each cell. The feedback regulation of Aallelic exclusion may depend upon both the inactivation of RAG activity as well as from the regulated alteration of accessibility to the heavy chain locus. Signals from the pre-B receptor presumably mediate the degradation of RAG-2. Since the pre-B receptor delivers a proliferative signal it is assumed to influence G1 progression by inducing cyclin D2 and activating cyclin dependent kinases such as CDK4 and CDK6. Activation of CDKs presumably mediates (among other things), the phosphorylation of the C-terminus of RAG-2 at threonine 490 (adjacent to proline 491). This phosphorylation event is probably critical for the polyubiquitination and proteasomal degradation of RAG-2. Although evidence linking RAG-2 degradation to allelic exclusion remains to be obtained, the evidence to the contrary is weak, and it should still be considered formally possible that heavy chain allelic exclusion depends in part on the induction of RAG-2 degradation by CDKs. A separate poorly understood event that occurs as a consequence of pre-B receptor signaling is an alteration of accessibility for V(D)J recombination specifically at the IgH locus.
Some mechanistic aspects of pre-B receptor signaling
There are two basic questions worth considering for pre-antigen receptors in general which we will discuss here in the pre-B context.
a) Is there a ligand for the pre-B receptor or is signaling constitutive and dependent primarily on assembly of the receptor?
b) What are the molecules and signaling pathways downstream of the pre-B receptor?
In spite of considerable effort over a decade, no ligand has so far been discovered for the pre-B or pre-T receptors though one might well be found in the future. The model suggesting that signaling might be constitutive is supported by experiments in which a truncated m chain has been found to drive cells through the pro-B to pre-B transition. Analagous experiments have been performed in the T lineage. Although in pre-B cells that are expressing surrogate light chains at high levels prior to light chain gene rearrangement the pre-B receptor has been described on the cell surface at low levels there is no hard evidence that it si actually expressed on the cell surface. Experiments in which the pre-T receptor has been held intracellularly have attempted to address this issue but might suffer from the problem that ER retention motifs compromise association with signaling chains. However even if surface expression is required, possibly for the receptor to be in the proximity of caveoli like DIFs in order to be in proximity to signaling molecules, it may well be that these receptors are activated in a ligand independent fashion.
Signal transduction via the pre-B receptor probably requires the activation of Src family kinases, Btk, and Syk. In the mouse Btk is not required at the pre-B stage. In man mutations in Btk are responsible for X-linked agammaglobulinemia, and most likely refelect the failure of pre-B receptor signaling and hence the failure to generate B cells in boys with this disease. Loss of the l5 homolog in man leads to a B cell developmental phenotype identical to that observed in X-linked agamaglobulinemia.
The induction of light chain gene rearrangement
Pre-B receptor signaling may be directly or indirectly responsible for a multitude of molecular events(Figure 6). As discussed above, this receptor may provide survival and proliferative signals, and may also be required for allelic exclusion at the heavy chain locus. In addition it may contribute to the efficient induction of k light chain gene rearrangement and also provide signals for the shut-off of surrogate light chain gene expression. It is clear that in the absence of pre-B receptor signaling that the k locus is at least minimally accessible for recombination. However signals from the pre-B receptor might cause the induction of nuclear factors which enhance accessibility at the k locus and thereby contribute to more efficient rearrangement in small preB cells.

Figure 6. Signal transduction by the pre-B receptor and its consequences
Signal transduction by the pre-B receptor directly or indirectly contributes to activation of the k locus. In vivo footprinting studies have revealed that the NFkB site in the k intronic enhancer is occupied even at the pro-B stage but as cells progress from the pro-B to the pre-B stage differences were noted in the set of transcriptional regulators bound to the 3’k enhancer (which is located about 10kB downstream of the Ck region).
Allelic exclusion and isotypic exclusion at light chain loci
Much less is understood about allelic exclusion at light chain loci (as compared with the H-chain locus, at whichour knowledge about this process remains pretty incomplete!). There is however growing evidence for the view that allelic exclusion at the k locus may be determined at the level of the intrinsic generation of accessibility. It has been noted that only one of the two k loci in any given B cell may become accessible perhaps because a limiting amount of an accessibility factor is synthesized and this factor can therefore open up only a single locus in a given cell.
How exactly isotypic exclusion (the inability of a l producer to rearrange k and vice versa) occurs is unclear. The stochastic choice of either k or l by a very limiting amount of an accessibility factor that favors one or the other locus is one explanation. A feedback process may also contribute to isotypic exclusion. The formation of a complete antibody molecule at the immature B cell stage contributes to the process of positive selection which will be discussed in some detail below. While "intense" signals through the BCReither lead to deletion or receptor editing, less well understood non-mitogenic signals delivered through the antigen receptor contribute to B cell maturation. One of the consequences of this maturation process is the feedback regulation of VDJ rearrangement. It is likely that maturation signals, in a poorly understood manner, shut off Rag gene expression (and probably inactivate accessibility factors as well) and that these events contributes to the process of isotypic exclusion.
Clonal deletion and receptor editing during B cell development in the bone-marrow
A proportion of the B cells that are generated each day express antigen receptors that are directed against self-antigens. B cells bearing receptors that are cognate for multivalent self-antigens (polysaccharides, membrane proteins, plasma membrane glycolipids) need to be either eliminated ot tolerized in some other manner. At the immature B cell stage, crosslinking of the B cell receptor by a multivalent ligand might induce signals that could lead to the induction of apoptotic death. Such a view has been supported by studies in which "monoclonal mice" have been generated which express specific immunoglobulin heavy and light chain transgenes that form a receptor directed against a multivalent antigen (a membrane anchored protein for example). Initial studies of this sort suggested that at the immature B stage a strong crosslinking stimulus could lead to the elimination of these B cells thus leading to the generation of tolerance. It has now become apparent that B cells which receive a strong crosslinking stimulus at the immature B cell stage may be given "another chance" or chances to reform themselves. They do this by undergoing further rearrangements primarily of light chain genes and also by presumably deleting their existing rearranged light chain genes. If a given B cell has already made a productive Vk to Jk rerrangement and is triggered to edit its receptor, the next Vk to Jk rearrangement that it makes will delete out and replace the previously rearranged VkJk unit. As a result this cell may now make a receptor that no longer is triggered by multivalent self and will both evade elimination as well as have the potential to be a useful citizen in a future pathogen directed immune response.
Receptor editing may also involve rearrangement of a l light chain in a cell that previously made a self-reactive B cell receptor that expresses k light chains. In this case the k locus might be deleted as a result of RS recombination using the RS/kDE element discussed above. The apparent importance of deletion of the pre-existing light chain rearrangemnt during receptor editing has been underscored by elegant studies in which the rearranged immunoglobulin heavy and light chain genes encoding an antibody directed against a specific MHC class I protein were "knocked-in" to the mouse germline. The VDJH and VJk units were therefore placed where they should be in the right chromosomal locations (in contrast to typical transgenes which are expressed from random locations in the genome). When the appropriate MHC class I gene was bred into this immunoglobulin "knock-in" mouse, immature B cells were not deleted but underwent receptor editing. Because the rearranged transgenes were in the "right" place it was possible to delete the self-reactive light chain gene in the process of generating a new L-chain rearrangement. If the original light chain were not deleted, the original self-recative B cell receptor would likely be still be around delivering unwelcome death signals.
From a mechanistic standpoint it is unclear whether a strong signal to a self-reactive B cell actually leads to the re-expression of Rag genes or merely prevents the cell from moving on to a stage when Rag genes are shut-off. The end-result is a cell that continues to rearrange its light chain genes. Indeed multiple light chain loci might have been generated by gene duplication and preserved during evolution primarily to facilitate the process of receptor editing. Receptor editing is primarily a light chain phenomenon. VH gene replacement is known to occur at the heavy chain locus where a new VH gene might recombine into an exiting rearranged gene by joining with a "cryptic" heptamer in the coding region of the previously rearranged VH gene.
Is deletional tolerance a "physiologically" relevant phenomenon in the B lineage? At present the jury is still out on this issue - the weight of the more recent evidence favors the view that multivalent self- antigens that can access the bone marrow probably induce tolerance by provoking antigen receptor signal mediated receptor editing. Other modalities of tolerance induction, such as anergy and follicular exclusion may be relevant at least for a subset of soluble self-antigens and these will be discussed in a later section in this chapter.
Positive selection II and peripheral maturation
For most students of the immune system positive selection of T cells during thymic development seems to make intuitive sense when one considers the need to select MHC restricted T cells. It has not been readily apparent why a positive selection phenomenon might be of relevance for recently generated B cells. Of the large number of B cells generated in the bone marrow every day (about 108) cells only about 10-15% actually make it to the periphery. Most newly generated B cells have a life-span that can be measured in days but a proportion of naive B cells become long-lived cells which may recirculate in the periohery for 6-8 weeks. The possibility has been raised in some studies that a proportion of newly generated B lymphocytes might in a non-random way (based on receptor specificity) be selected to develop in the periphery and to become long lived B cells.
It is now clear that at the immature B cell stage, in addition to negative selection or receptor editing, B lymphocytes are also positively selected. In mice in which a mutation inthe cytoplasmic tail of Iga was engineered, B cell development occurred in the bone marrow but emigration to the periphery appeared to be severely compromised. Inan antigen specific "knock-in" mouse model in which B cell receptor genes were conditionally deleted in the periphery, the maintenance of these peripheral B cells was severely compromised. Signals from the B cell receptor are therefore required both for the emergence of B cells in the periphery as well as for maintenance. It remains unclear at this time whether there is actually a signal delivered vis the B cell receptor that drives emigration from the bone marrow or whether low-levelantigen receptor signals facilitate follicular entry in the periphery. It seems likely that folliciular entry requires a minimal signal from the BCR (while stronger signals from the BCR may impede this process as discussed below), as well as a chemokine derived signal. Downstream of the B cell receptor, it appears that Syk might play a role in the peripheral emigration/follicular entry step whereas Btk is required for the maintenance of long-lived peripheral B cells.
Immature B cells (stage E) emerge from the bone marrow beteween 2-3 days after they receive the pre-B receptor signal in stage C/C’ and emerge in the spleen as a IgMhiIgDlo population (Fraction F III, according to categories described by Hardy and colleagues). The splenic IgMhiIgDlo population contains both recently generated bone-marrow derived B cells as well as a distinguishable "older" subset of B cells known as marginal zone B cells. Recently generated Fraction III cells which emerge in the spleen are distributed initially in the T cell zone and in the red pulp. Low-level BCR signals which require Syk activity might contribute to the entry of these cells into the follicles. The newly generated Fraction III cells mature in the periphery over the next 1 to 3 days into IgM high, IgD high (Fraction II) B cells. In a few days these Fraction II cells in turn mature into IgDhigh, IgM low (Fraction I) B cells which represent the most mature peripheral B cell population. Fraction II and Fraction I cells express high levels of CD23 and acquire the ability to recirculate, migrating constantly between follicular areas in the spleen and other peripheral lymphoid organs. Indeed in just a day after bone-marrow cells enter the spleen (soon after they enter Fraction II in the follicles), they acquire the ability to recirculate and seed lymph nodes.
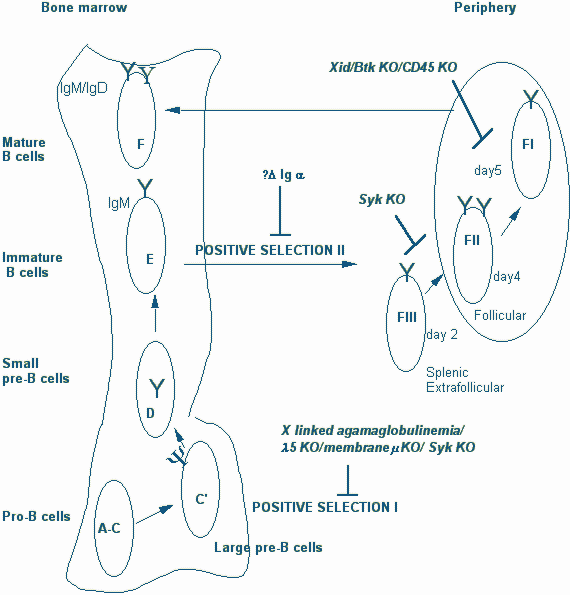
Figure 7. Checkpoints for positive selection during B cell development
In secondary lymphoid organs IgDhi (Fraction II and I) B cells reside largely in lymphoid follicles which may in some unknown way contribute to their extended life span. It is now clear that in the absence of Btk signals, cells that enter Fraction I are unable to become long lived B cells and rapidly die. Btk derived signals are necessary for the generation of the long-lived pool of naive recirculating peripheral B cells. The steady-state level of Fraction I cells is markedly diminished in the absence of Btk. This fraction is also decreased in the absence of CD45 which is required for Src family kinase activation and therefore presumably for Btk activation as well. The proportion of Fraction I cells increases in the absence of CD22 (which functions as a negative regulator of the BCR). It is considered likely therefore that non-mitogenic, low-intensity signals are delivered by the BCR via the Btk pathway and that these signals are essential for the maintenance of long-lived naive B cells.
What drives positive selection? Does the B cell receptor signal "tonically" as has been suggested or is it triggered in a non-mitogenic fashion by cross-reactive self antigens? Is the "tonic" signal, merely generated to tell a B cell that its bone-marrow developmental program is complete and that it should now shut off its VDJ recombination machinery and move out to where the real action is going to be? If the signal is tonic it might be reasonable to suggest that this process is NOT positive "selection" but representsa set of maturational events. It could be that what is being selected is an appropriate fit between heavy and light chain. No conclusive answer is available at present for any of the questions raised above. However it is conceivable that in the absence of a pathogen induced challenge this process is driven by available antigen and that this selection process actually evolved for a defense related reason. It is conceivable that positive selection is generally driven by non-crosslinking self -antigens and that in a viremic or otherwise infected host non-crosslinking foreign antigens may target emerging B cells and bias the peripheral B cell repertoire towards the pathogen that requires to be targeted. This could potentially result in there being more of the right kind of b cells in the periphery to drive T-dependent responses that are likley to be of protective value.
In summary, signals from the B cell receptor are required for the maturation of naive immature B cells into recirculating follicular long-lived B cells. The most mature long-lived population is made up of IgDloIgMhi cells. This maturation process may be referred to as Positive Selection II (Figure 8) to distinguish it from Positive selection I. These signals must represent non-mitogenic trophic "tickles" rather than the full fledged stimulation associated with cross-linking antigens. While there is evidence that supports the notion of cell loss somewhere between the bone-marrow and the spleen, this is based on very indirect and potentially error-prone estimations of daily bone-marrow production. There is no hard evidence to suggest that only a proportion of the emerging B cell repertoire is selected positively or negatively at the immature B cell stage. It is likely that low-intensity BCR signals delivered via the Syk tyrosine kinase assist in follicular entry (strong BCR signals as we will discuss later may do the opposite). Signals delivered via Btk may be required for peripheral B cell maintenance and therefore for the generation of long-lived B cells.
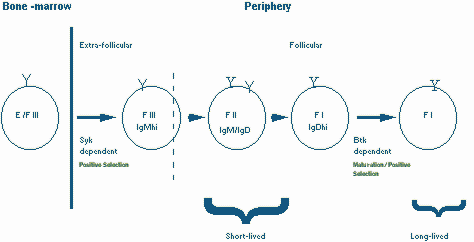
Figure 8. Positive selection II: Immature B cells are positively selected to exit the bone marrow and enter lymphoid follicles by Syk dependent mechanisms and to mature into long-lived B cells by a Btk dependent pathway
Peripheral B cell populations and their maintenance
Peripheral B cell first emerge in the spleen. As mentioned above soon after entering primary follicles (follicles which lack germinal centers) they acquire the ability to recirculate and move from one follicular site to the other. this migratory behavior enables them to be available for any cognate antigen that might emerge at a given site and to be in proximity to recirculating T cells that might provide help.The following B cell populations may be identified in the spleen.
1. Recently generated B cells which are IgMhi IgDlo/- CD23- and CD21 lo. These newly generated naive B cells cells, sometimes referred to as virgin B cells, form a portion of Faction III, and they express high levels of CD24/HSA. These cells may be found in the red pulp of the spleen and in the T cell areas (the periarteriolar lymphoid sheath in the spleen).
2. Follicular B cells are derived from the recently generated Fraction III cells. These cells colonize lymphoid follicles, express lower levels of CD24/HSA, have the ability to recirculate, and initially have a IgMhi IgDhi CD23+ phenotype (Fraction II). They mature very rapidly into IgMlo IgDhi CD23+ cells (Fraction I). These naive B cells are variously referred to as mature B cells, or follicular B cells, or recirculating B cells.
3. Marginal Zone B cells. The marginal zone surrounds the follicular area and is populated by B cells that are IgMhi, IgD-, CD23-, and CD21hi. These cells can be distinguished from newly generated fraction III cells by virtue of their higher levels of CD21. The marginal zone also contains marginal zone macrophages, and a distinct population of marginal metallophilic macrophages. Marginal zone B cells are cells that are "ready to go" and can present antigen very efficiently, and can also differentiate into plasma cells in a matter of hours. These non-recirculating cells probably represent an unusual memory population generated in the spleen (and in lymph nodes in man), by multivalent antigens such as complement coated bacterial polysaccharides which are made available on the surface of the specialized macrophages in the area. Marginal zone B cells are largely found in the spleen, and probably account for the critical role played by the spleen (as opposed to other peripheral lymphoid sites) in responses to blood-borne carbohydrate antigens. Marginal zone B cells have been considered to represent a separate lineage.
While recently generated peripheral B cells might require non-mitogenic Syk signals to enter follicles, entry (and repeated re-entry) into follicles may also require signals delivered via follicular chemokines that serves as chemoattractants for B cells. One of these is a C-X-C chemokine known as BLC (B lymphocyte chemoattractant) or BCA-1(B cell attracting chemokine-1) which signals via a chemokine receptor that is present on mature B cells known as BLR-1 (for Burkitt’s Lymphoma Receptor-1) now reclassified as CXCR5. BLC was first described in mouse tissues and BCA-1 is the human homolog of BLC. Another B cell chemoattractant is SDF-1 which activates CXCR5 and is, as we have discussed earlier, also essential for the expansion of pro-B cells in the bone-marrow. BLR-1/CXCR5 may be more critical for migration into follicles in the spleen and Peyer’s patches (since the BLR-1 knock-out mouse fails to develop follicles at these locations) while CXCR4 may be crucial at other sites. Recirculating B cells emerge from the marginal sinus or from the central arteriole, migrate through the outer T cell zone and if they are not challenged by antigen enter the adjacent follicles.
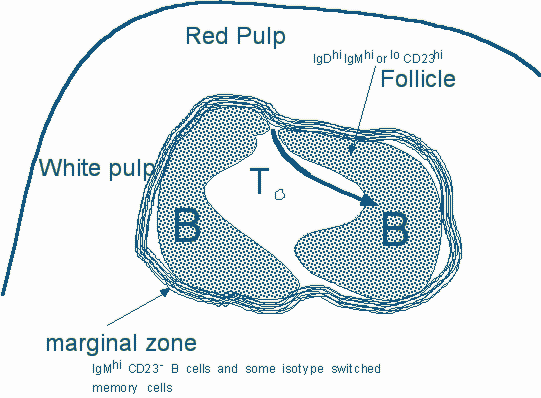
Figure 9. A schematic view of a segment of the spleen. The white pulp is made up of follicles which are B cell areas and the periarteriolar lymphoid sheath which represents a T cell zone. Both BLR1/CXCR5 and CXCR4 (the receptor for the SDF-1 chemokine) participate in the entry of B cells ino follicles
m and d heavy chains are generated from a rearranged Ig locus by RNA processing
Immature B cells express no IgM and as they mature they express some IgD in fraction III and mature in the follicle into cells expressing high levels of IgD. After activation the cells revert to an IgM hi phenotype. IgM and IgD are generated from long transcripts initiating upstream of a rearranged VDJ unit and extending across m and d constant regions on this chromosome. The ratios of IgM and IgD at different stages of peripheral differentiation are regulated at the level of RNA processing (Figure 10). While polyadenylation site selection most likely plays a critical role in this process, the molecular basis for the regulation of IgD during development is poorly understood.
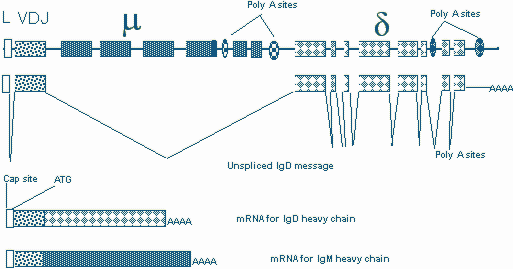
Figure 10. m and d heavy chains are generated by RNA processing of a long transcript.
Peripheral tolerance and the putative function of IgD
Cells expressing IgD represent mature peripheral B cells and are therefore not as prone to being tolerized as immature B cells in the bone-marrow. The presence of IgD has therefore long been considered as a hallmark of maturity but we remain in the dark as to whether the IgD molecules on the cells surface function in a distinct way from IgM. IgD knockout mice have been generated but few clues have been obtained from these mice as to the functional significance of IgD. there is no firm evidence to indicate that IgD induces signaling differently from IgM. The function of IgD remains an enigma.
T-dependent and T-independent pathways of B cell activation
B cells can be triggered by two broad categories of antigen to differentiate along two distinct pathways. Multivalent antigens, exemplified by bacterial polysaccharides, can directly trigger B cells with little or no T cell help. While this category of antigens can invoke an immune response and antibody secretion the B cell is not generally triggered to go through the processes of isotype switching and affinity maturation. As aresult the antibodies generated by a T-independent antigen tend to be mainly of the IgM clas and of relatively low affinity.
Protein antigens are internalized by B cells (by receptor mediated endocytosis) cleaved into peptides and specific peptide-MHC class II complexes are presented to cognate T cells. An activated T cell may trigger a B cell by a number of means the most critical receptors on the B cell being CD40 and the IL-4 receptor. T-dependent responses result in the formation of germinal centers (described below) in secondary lymphoid organs and it in these specialized expansions of B cells that the processes of isotype switching and somatic mutation occur.
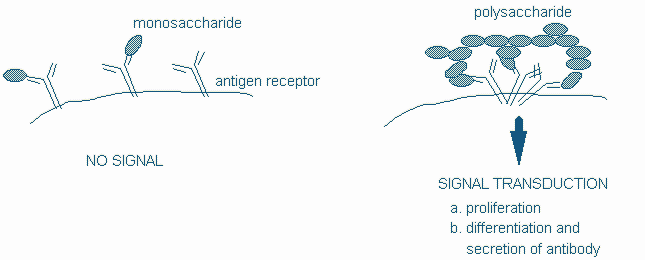
Figure 11: Crosslinking of the BCR provides a T-independent signal
Diversification and recombinational events that occur in germinal centers: somatic mutation and isotype switching
Mature B cells may encounter cognate protein antigens (T-dependent) or their receptors may be crosslinked by multivalent T-independent antigens. Protein antigens are bound by the antigen receptor, processed and presented in an MHC class II context to helper T cells. The CD40 ligand on activated T cells delivers a growth signal to B cells via the CD40 molecule on B cells. Additional signals are provided by T cell derived cytokines. T dependent signals are delivered in the germinal centers of lymph nodes and contribute to clonal expansion, the initiation of isotype switching and somatic mutation. In patients with X-linked hyper IgM syndrome, T-B collaboration is abrogated because of an inherited absence or defect in the CD40 ligand.
Figure 12. T-dependent signaling
Isotype switching
While signals via CD40 are essential for expansion, somatic mutation and isotype switching, specific signals from distinct cytokine receptors may influence isotype switching from IgM to specific heavy chain loci. The generation of interleukin-4 by helper T cells may for instance help drive class switching to IgE, while a high local concentration of TGF-b may drive switching to IgA. Signal transduction leads to the transcriptional induction of DNA binding proteins which identify switch sites upstream of heavy chain constant regions and help mediate a deletional recombination event which brings the rearranged VDJ unit from its location adjacent to the m locus to a site next to the heavy chain locus targeted for switching.
Upstream of each constant region is a region of DNA known as a switch (S) region. S regions contain multiple repeats of GAGCT and TGGGG. Just upstream of each switch region is an I region promoter and an I exon. When a heavy chain isotype is targeted for class switching, a transcript is initiated from the I region 5' to the targeted constant region. The transcript reads through the I exon, the switch region and the constant region exons and is terminated and polyadenylated at the 3 ' end of the constant regions. This transcript is spliced (removing the switch region and other introns). It has been postulated that the spliced RNA might be a structural component of the switch recombinase. Very little is known at present about this recombinase.
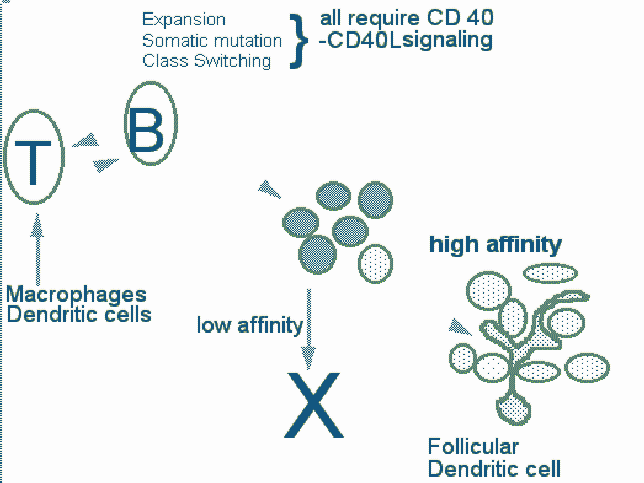
Figure 13. An outline of the process of affinity maturation
Cell selection in germinal centers and affinity maturation
Somatic mutation occurs in the dark zone cells in germinal centers of lymph nodes. As a result of this process a number of B cell clones with altered affinity for the original immunogen are generated. Clones with the highest affinity are selected and expand to form germinal centers. In this process, V regions and flanking sequences are mutated. The mutational process starts from the 5' end of the rearranged V gene. The 3' border of the segment that is mutated is variable, and mutation can spread into the adjacent intron. There is a strand bias in the process which has led to the enunciation of transcription/replication based models for somatic mutation. This processs can be studied in transgenic mice. Studies using transgenes have indicated that there are hot spots for mutation. In the case of k transgenes, the 3 ' enhancer is not required for somatic mutation to occur and other promoters can replace the V gene promoter. However the presence of the intronic k enhancer is essential for mutation to be seen.
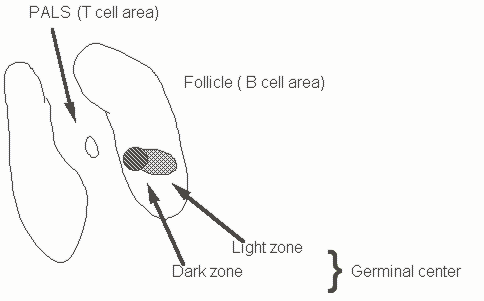
Figure 14. Outline of B and T cell zones and germinal centers in secondary lymphoid organs
These and related findings have led to a "Transcription coupled repair" model for somatic mutation.
Selected Reviews
Goodnow, C. C. (1996). Balancing immunity and tolerance:deleting and tuning lymphocyte repertoires. Proc.Natl.Acad.Sci. USA 93, 2264-2271.
Pillai, S. (1999). The Chosen Few? Positive Selection and the generation of Naive B lymphocytes. Immunity 10, 493-502.
Rajewsky, K. (1996). Clonal selection and learning in the antibody system. Nature 381, 751-758.
Objectives / Study Questions
1. Describe the pre-B receptor and outline its functional roles during B cell development. Distinguish between allelic exclusion and isotypic exclusion.
2. What is receptor editing? At what stage of development is clonal deletion of B cells presumed to occur?
3. What are T-dependent and T-independent antigens?
4. What immunoglobulins are expressed on the surface of naive follicular B cells? What are the mechanisms involved in the expression of these molecules?
5. What is the major site at which marginal zone B cells are found? What do these cells do?
6. What are B-1 cells?
7. How does isotype switching occur?
8. What is the germinal center reaction. What changes might a B cell undergo in a germinal center?
9. What functions are mediated by CD40 on B cells?