Developing new tools to take the research forward. Glycosylation has long been the focus of Imperiali’s work. “We will use any method or tool we can to try to understand this very complex biological process, which involves the enzyme-catalysed modification of proteins with complex carbohydrates to alter their structure or function.”
N-linked Glycosylation
Asparagine-linked (N-linked) glycosylation is an essential protein modification that is associated with all domains of life. N-linked glycosylation occurs via a complex and integrated sequence of enzymatic transformations that result first in the assembly of a key membrane-bound polyprenyl-pyrophosphate-linked glycosyl donor. The pathway culminates with glycosyl transfer to protein, which is catalyzed by the integral membrane enzyme oligosaccharyl transferase (OTase). N-linked glycans play profound and critical structural and functional roles in living organisms.
Current research in the Imperiali group seeks to provide inhibitors as tools to probe the roles of glycosylation in pathogenic bacteria, methodological approaches for investigating the coordinated action of glycosylation pathway enzymes that function at the membrane interface, and active, monomeric OTases from prokaryotic sources for detailed biochemical and biophysical analysis.
Discovery of prokaryotic N-linked glycosylation inhibitors
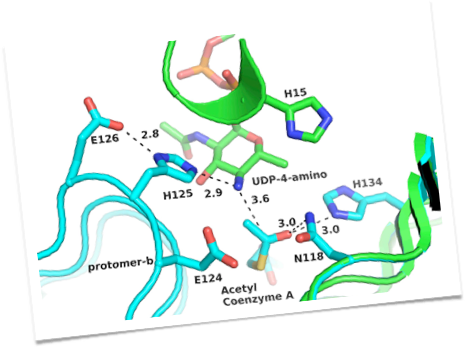
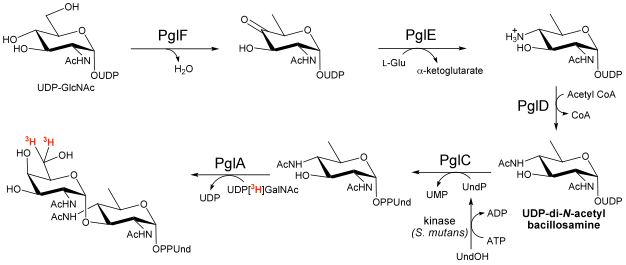
Unusual modified saccharides, such as di-N-acetyl bacillosamine (diNAcBac), pseudaminic acid and N-acetyl mannuronic acid are essential elements of N- and O-linked glycoproteins and the lipopolysaccharide cell wall components in several eubacterial pathogens. N-linked glycoprotein biosynthesis in Campylobacter jejuni involves the intermediacy of UDP-diNAcBac and undecaprenyl-PP-diNAcBac and compelling evidence is accumulating that the genesis of these intermediates in implicated in the microorganism pathogenesis.
In our lab the biosyntheses of several unusual sugars have been reconstituted in vitro, and now we are developing a multienzyme kinetic assay for the discovery of selective small molecule inhibitors of the enzymes that convert UDP-N-acetylglucosamine into Und-PP-diNAcBac. These enzymes share a common theme in that all four act on UDP-sugar (pyranose) substrates. Compounds that are identified in the multienzyme assay will be studied in detail to identify the specific enzyme target, to optimize activity and selectivity, and to investigate cell permeability. The activity of any promising inhibitors will be tested in cellular C. jejuni assays and in vivo in colonization infection model systems in the Szymanski lab (University of Alberta). Ultimately, inhibition will also be tested in homologous enzymes in other pathogens including Neisseria gonorrhoeae and N. meningitides. The availability of such inhibitors should provide new and complementary tools for studying the roles of cell surface glycans in virulence and pathogenicity
Uncovering the integrated action of prokaryotic N-linked glycosylation pathway enzymes
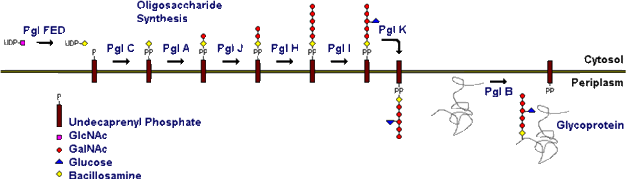
N-linked protein glycosylation in all organisms involves the sequential action of multiple enzymes, which act at a membrane interface on highly lipophilic substrates to afford complex polyprenyl-pyrophosphate-linked oligosaccharides. We are investigating the physical interactions and the sequential catalytic activities of a series of N-linked glycosylation pathway enzymes in the context of the C. jejuni system, which include the PglC, PglA, PglJ, PglH, and PglI enzymes. Fundamental issues that are being addressed concern the involvement of the membrane bilayer in the sequential pathway transformations and the role of the hydrophobic undecaprenyl moiety that is a common feature of the enzyme pathway substrates. A central aspect of the research is the application of phospholipid bilayer mimetics for studying the membrane-associated enzymes in the pgl pathway in a native-like environment that is amenable to biophysical and biochemical analysis.
Monomeric oligosaccharyltransferases from prokaryotic organisms
OTases catalyze a mechanistically unique and selective glycosylation of the asparagine amide side chain of nascent or folded proteins. In contrast to the eukaryotic OTases, which are multimeric membrane-bound complexes, the prokaryotic OTases appear to be monomeric. In order to pursue detailed biochemical and biophysical studies on a functional OTase, it is essential that we identify a tractable monomeric OTase with excellent expression and stability properties. Archaeal protein homologs have proven to be very valuable for the analysis of protein structure and function due to improved expression in heterologous expression systems and advantageous stability properties. The current goal of these studies is to identify a monomeric archaeal OTase that shows high expression in a practical heterologous system, stability upon purification and on storing, and suitability for assay development and kinetic analysis. Detailed biochemical and biophysical studies will be pursued once a suitable OTase homolog has been identified.