Chemical Energy
Storage
Chemical Energy
Storage
Home / Research / Energy Storage / Chemical Energy Storage
Organometallic Compounds for Storing Sunlight Energy
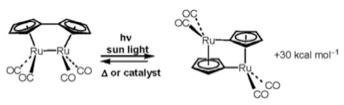
We are at present using computation to understand how and why this reaction takes place, and also how to engineer the efficiency of the reaction in order to increase its ability to store energy. In addition, we are investigating a number of other such molecules that fall into the same class of sunlight-to-chemical energy storage. We’re also exploring pathways for utilizing such molecules on large scales, which involve appropriate matrix materials (which in turn impact its chemical properties).


Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge MA 02139-4307
