Hydrogen Storage
Home / Research / Energy Storage / Hydrogen Storage
Tuning Hydrogen Storage Desorption Energies: The Crucial Importance of High-Accuracy Calculations
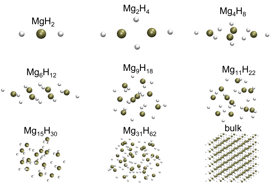
Recently it has been shown that it is possible to tune the desorption energy of a material simply by making into nanoparticles. For example, Wagesmans and others have shown theoretically that Mg clusters can be tuned to much lower H2 desorption energies compared to bulk for sizes less than about 20(MgH2). As we move forward in our own work to explore other mechanisms for tuning the desorption energies, including nanoparticles but also alloying, we note that one must use caution in making predictions for such energetics using the “standard” density functional theory approaches. In fact, for the MgH2 system, we’ve shown using highly accurate QMC methods that the DFT approaches fail to describe the desorption behavior and even that there is a size-dependence to the DFT error.
In the coming months we’ll present new results for H2 storage materials and also details of the QMC calculations that help explain how and why the DFT methods may fail for such predictions.


Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge MA 02139-4307

