The periodic monotone of bulk electronic and structural properties comes to an end at the surface of a material. A rather abrupt change in the topology and the crystal potential at the surface produces many interesting physical and chemical properties comprising surface phenomena. For instance, while atoms in the bulk of a solid are happily bonded in all directions to their neighbors, bonds at the surface often remain unsaturated leading to reconstructions, enhanced chemical reactivity, and interesting electronic properties. Since most of our interactions with materials occur via surfaces, the study of these also forms one of the most fascinating topics of current research and one of great practical interest as well.
Surface at nano-scale
Surface chemistry and physics become increasingly more important for understanding materials properties as the material size becomes smaller. At a nano scale, the surfaces can even dominantly determine the materials properties, playing the critical role together with the confinement effects. It is therefore important to understand both structural and electronic roles of the material surfaces for designing/investigating the nano-materials.
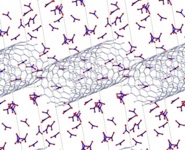
Carbon Nanotube Surface Chemistry
Ever since their discovery in 1991, carbon nanotubes (CNT) have been one of the most intriguing class of nano-materials for technological applications. While their electronic properties have been exhaustively investigate
d both experimentally and theoretically, CNT's chemical reactivities remain less well-understood. CNT surface is known to be rather inert toward chemical reactions despite its importance in integrating bio/chemical functionalities into future CNT-based optoelectronics. Collins and co-workers have recently shown that the CNT oxidation efficiently induces chemical reactions at surface by monitoring the discrete change of CNT electrical conductance [Science, 315,77 (2007)]. In order to understand this observation at an atomistic level, we are investigating how the CNT oxidation influences the surface reactivity of the CNT toward molecules, in collaboration with the Collins Group at U.C. Irvine.
Dissolution of Hydroxyapatite surfaces
Hydroxyapatite (Ca10(PO4)_10(OH)2) belongs to a class of naturally occurring minerals called apatites that have shown a lot of promise as candidates for bio-materials. Hydroxyapatite (HA) itself occurs as one of the main components of mammalian bones and teeth enamel and for that reason has been the subject of mu
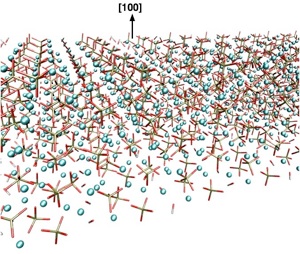
ch past and current research. The demineralization of enamel under acidic conditions essentially happens due to the dissolution of the HA component and leads ultimately to dental caries. Fluoride treatment has been known to lead to strengthening of tooth enamel by preventing dissolution of HA. However, the microscopic origins of the anti-cariogenic properties of fluoride ions are as yet unclear. In particular, the role played surface defects, kinks and steps in the reactions is yet to be outlined. Using dynamic atomic-force microscopy (d-AFM), the group of Prof. Seung-wuk Lee at the Department of Chemical Engineering at Berkeley, has been able to study the dissolution of HA (100) surfaces in acidic conditions by following the growth of etch-pits under varying fluoride concentrations. Motivated by the extremely interesting results of these experiments, we are presently carrying out density-functional theory studies of the clean (100) surface as well as the same surface with the steps observed in the d-AFM studies. The combination of high quality experiments and highly accurate theory can lead to great insights into the fundamental mechanisms behind the anti-cariogenic properties of fluoride ions.