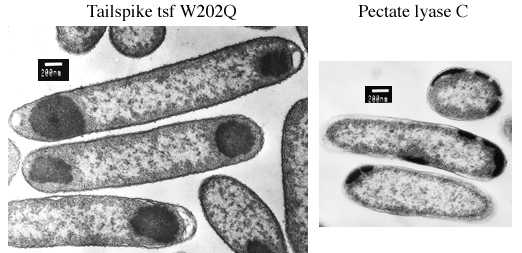

During synthesis at 37°C, tailspike polypeptides containing
temperature-sensitive folding mutations aggregate quantitatively in
the cytoplasm of host E. coli cells. The aggregated
polypeptide chains accumulate as electron-dense inclusion bodies
visible at one or both poles of the cells in these electron
micrographs.
The right-handed parallel ß-coil motif was discoverd in 1993 when the crystal structure of pectate lyase C was solved (Yoder MD, Keen NT, Jurnak F (1993) Science 260:1503-1507). This enzyme is a virulence factor secreted by Erwinia chrysanthemi, a soft-rot plant pathogen. In E. coli, recombinant pectate lyase C is targeted to the periplasmic space by its native signal peptide, where at low temperature it accumulates in the processed, mature form. At 37°C, however, pectate lyase C aggregates and accumulates as electron-dense inclusion bodies that appear to be excluded from the cytoplasm. The location of pectate lyase C inclusion bodies at the cell periphery suggests that they form in the periplasmic space.
Sample processing and electron microscopy were performed by Patricia Reilly at the Biomedical Electron Microscopy facility at MIT. The plasmid encoding the pectate lyase C precursor was kindly provided by Noel Keen and Frances Jurnak.
Betts, SD & King, J (1998) Cold rescue of the thermolabile tailspike intermediate at the junction between productive folding and off-pathway aggregation. Protein Science 7(7):1516-1523. (Go to the article)
Betts S, Haase-Pettingell C, King J (1998) Mutational effects on inclusion body formation. Advances in Protein Chemistry 50:243-264.