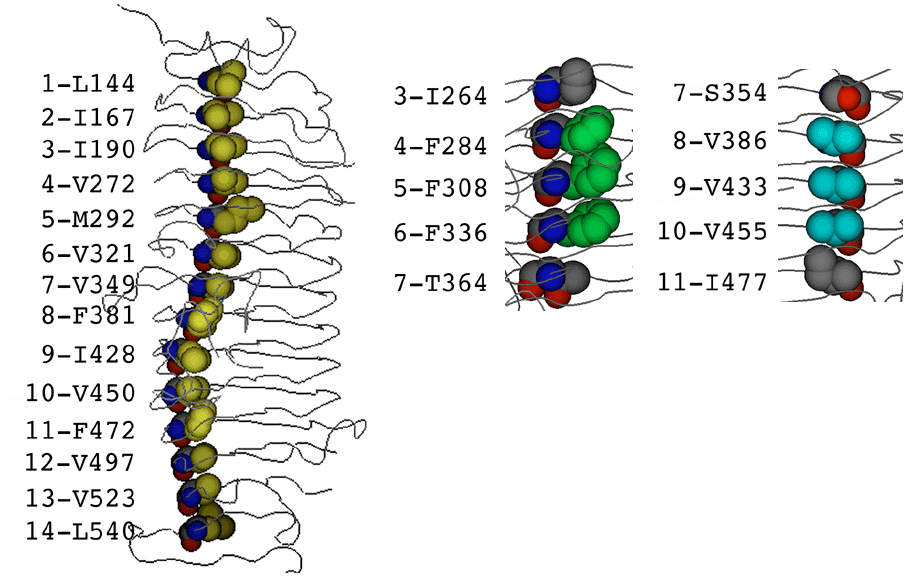
A prominent feature of parallel ß-helix domains is the cupped stacking of buried side chains. Residues in equivalent positions in consecutive coils are often oriented identically. This results in the formation of closely packed stacks of either polar or nonpolar side chains. The side-chain stacks provide the structural basis for generating sequence alignments of the thirteen contiguous coils between residues 143-540 in the tailspike protein (see below).

Abola, E., Bernstein, F.C., Bryant, S.H., Koetzle, T.F., Weng, J. (1987) Protein Data Bank. In Crystallographic Databases: Information Content, Software Systems, Scientific Applications (Allen, F.H., Bergerhoff, G., Sievers, R., Eds.), pp.107-132.
Steinbacher, S., Seckler, R., Miller, S., Steipe, B., Huber, R., Reinemer, P. (1994) Crystal structure of P22 tailspike protein: Interdigitated subunits in a thermostable trimer. Science 265, 383-6.