| Introduction | The Pharmaceutical Pipeline | POPI Personnel | POPI Research | POPI Education | POPI and Public Policy |
Annual Report of Activities, 1999-2000
September, 2000
The MIT Program on the Pharmaceutical Industry (POPI) was founded in 1991 with the support of the Alfred P. Sloan Foundation. POPI is a research and education program focused on understanding the structure and dynamics of the global pharmaceutical industry. Our research concentrates on the firms that make up the industry, as well as their customers and government bodies providing oversight of the industry. We educate doctoral students to teach and conduct research in areas related to the industry. Through our research, POPI seeks to provide insights that can contribute to the discovery, development, and marketing of new drugs that add value to health and the delivery of medical care.
The Programís multidisciplinary approach involves faculty from the MIT Schools of Engineering, Science, Humanities and Social Sciences, the MIT Sloan School of Management, and other institutions. Graduate students in departments across MIT receive POPI support and play an important role on POPI research teams. Since 1994, a number of pharmaceutical firms have helped fund POPI research as corporate sponsors, and have provided data and insights to POPI researchers.
| Top of Page | Homepage |
POPI research spans a wide spectrum of pharmaceutical industry activity. The pharmaceutical ďpipelineĒ helps inform our understanding of the activities of firms in the industry. We use it as a tool for organizing our multidisciplinary research efforts, which correspond to the interrelated steps, or stages, of the pipeline. Our research falls into several overarching research areas: drug discovery and development, manufacturing, and the business of pharmaceuticals.
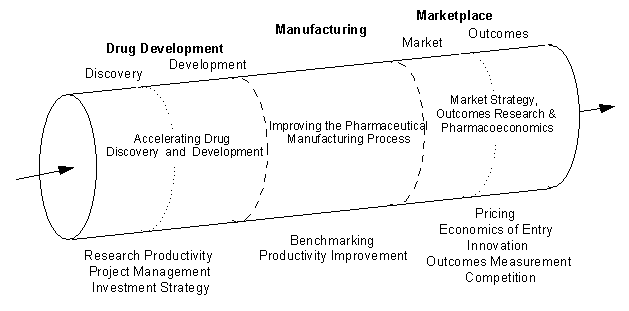
In the area of drug discovery and development, POPI research teams have explored research productivity, project management, investment risk and return, and new designs for clinical evaluation. Our manufacturing research (now funded separately through the Consortium for Advances in the Manufacture of Pharmaceuticals, CAMP), explores benchmarking, cost impacts, and other issues of concern to firms. And research teams looking at the business of pharmaceuticals explore the economics of new markets, technology transfer, outcomes research, competitive strategies, and a range of public policy concerns.
POPI brings together a multidisciplinary mix of MIT faculty from the Schools of Engineering, Science, Humanities and Social Sciences, the Sloan School of Management, and the Harvard-MIT Division of Health Sciences and Technology.
MIT doctoral students in economics, management, and operations research are encouraged to complete doctoral theses with POPI faculty on topics related to pharmaceuticals. Other MIT masterís and doctoral degree candidates in a variety of departments ó including biology, chemical engineering, and management ó enroll in our classes and work on POPI research projects.
POPI also involves outside collaborators in research projects, and maintains ongoing contact with former doctoral program students who continue to work in or conduct academic research into the pharmaceutical industry. In addition, POPI has a post-doctoral associate in the Sloan School of Management and a post-doctoral associate in the Department of Biology. Each plays an important role in POPI research.
Faculty
POPI faculty are realizing new opportunities for professional development through their association with POPI. Successful professional development of faculty in the POPI context involves the ability to conduct rigorous research that meets the criteria for excellent scholarly work in a given academic discipline, passes the test of peer review in a given discipline, is on a topic relevant to the pharmaceutical world and that is relevant, in particular, to public policy needs.
The following are current POPI faculty at MIT (CV's available upon request):
Thomas J. Allen, Ph.D., Howard W. Johnson Professor of Management, Sloan School of Management; Co-Director of POPI
Deborah G. Ancona, Ph.D., Seley Distinguished Professor In Management
Arnold I. Barnett, Ph.D., George Eastman Professor of Management Science
Ernst R. Berndt, Ph.D., Louis B. Seley Professor of Applied Economics
Charles L. Cooney, Ph.D., Professor of Chemical & Biochemical Engineering; Co-Director of POPI
Sara Fisher Ellison, Ph.D., Senior Lecturer in Economics
Stan N. Finkelstein, M.D., Senior Research Scientist; Co-Director of POPI
Robert G. Gibbons, Ph.D., Sloan Distinguished Professor Of Management
Rebecca M. Henderson, Ph.D., Eastman Kodak LFM Associate Professor of Management
Ralph Katz, Ph.D., Principal Research Associate
Stewart C. Myers, Ph.D., Gordon Y Billard Professor of Finance
Robert S. Pindyck, Ph.D., Mitsubishi Bank Professor In Economics & Finance
ChoKyun Rha, Ph.D., Professor, Biomaterials Science & Engineering Laboratory
Robert H. Rubin, M.D., The Gordon & Marjorie Osborne Associate Professor, Harvard-MIT Division Health Sciences & Technology
Anthony J. Sinskey, Sc.D., Professor of Microbiology
Scott Stern, Ph.D., Ntv Career Development Assistant Professor of Management
Glen L. Urban, Ph.D., Dai-Ichi Kangyo Bank Professor of Management
Lawrence M. Wein, Ph.D., Digital Equipment Corporation LFM Professor of Management Science
Graduate Students
There are currently six graduate students funded in part by POPI.
Pierre Azoulay is a doctoral student in strategic management at the Sloan School of Management. His research interests include the question of whether specific human capital matters for organizational design, using drug development as the ďlaboratoryĒ for exploration. His work illustrates how shifting the boundaries of the firm can help provide high-powered incentives for learning and cooperative activities, which are critical determinants of project-level performance. Specifically, Azoulay looks at Contract Research Organizations (CROs), to which pharmaceutical firms have outsourced much of their clinical trials. He draws inferences regarding why performance is higher when pharmaceutical firms conduct this work in-house. Azoulayís faculty advisors are Rebecca Henderson, Robert Gibbons, Ernst Berndt, and Scott Stern.
Henrik Bresman is a doctoral student in the organization studies group at the Sloan School of Management. His research has focused on how a product development team operating in a rapidly changing environment integrates an external technology that constitutes a core component of the new product. The study uses the setting of in licensing in pharmaceutical drug development. Bresmanís faculty advisors are Deborah Ancona and Thomas Allen.
Astrid Andrea Dick is a doctoral candidate in economics. Her research has focused on the consequences of physician authority on pharmaceutical prescribing, where she has helped uncover substantial variation in the degree to which physician prescribing is concentrated (i.e., that some physicians prescribe a more diverse portfolio of drugs than do others), and that this concentration is correlated with observable drug characteristics ó in particular, high levels of advertising, low prices, and high market share. Her faculty advisor is Scott Stern.
Jeffrey Furman is a Ph.D. candidate in economics. His research looks at the effects of location on the organization of drug discovery. Specifically, he is exploring the extent to which factors that vary across geographic regions, including public policy decisions and the nature and extent of knowledge spillovers, explain heterogeneity in the organization of firms and research laboratories in the pharmaceutical industry. Furmanís faculty advisors are Rebecca Henderson, Iain Cockburn, and Scott Stern.
Margaret Kyle, a doctoral candidate in economics, studies the implication of patent expiration on firms in the pharmaceutical industry. She has been on a research team exploring the marketing efforts and pricing behavior of firms in the market for H2-antagonist drugs that have experienced patent expiration or that have implemented prescription-only to over-the-counter switches. Kyleís faculty advisors are Scott Stern, Ernst Berndt, and Rebecca Henderson.
Jason Verner recently graduated from the Sloan School of Management with an MBA degree. His research interest is workplace productivity. As part of research into at-work productivity among employees with mental disorders has involved the analysis of a database of objective (daily) productivity metrics for 2,000 claims processors at a major insurance company, along with their medical claims. He has also looked at asthma among dependents of workers and found that mothersí work productivity declines when their dependent children have asthmatic episodes. Vernerís faculty advisors are Ernst Berndt and Stan Finkelstein.
Post-Doctoral Associates
POPI supports two post-doctoral associates at MIT.
G.K. Raju serves as the manager of POPIís Consortium for Advances in the Manufacture of Pharmaceuticals (CAMP). He is also available to other POPI projects as a consultant on pharmaceutical production issues. Raju earned his MBA from the Sloan School of Management and a Ph.D. in chemical engineering from MIT.
Sarah Stallings manages the new POPI research project on ďScience and Technology as Drivers of Change in the Pharmaceutical Industry,Ē which explores the competitiveness of firms in the industry and the relationship to firmsí ability to incorporate new science and technology into their operations for discovery, development, clinical trials, manufacturing, marketing, and distribution. Stallings earned a Ph.D. in molecular biology from Yale University.
POPI Outside Collaborators
In addition to MIT faculty, POPI works with collaborating faculty from several other institutions:
Howard L. Bailit, D.M.D., Ph.D., Professor of Community Medicine, University of Connecticut Health Center
Iain Cockburn, Ph.D., Professor, School of Management, Boston University
William Crown, Ph.D., Vice President, The Medstat Group
Richard G. Frank, Ph.D., Professor, Department of Health Care Policy, Harvard Medical School
Kenan E. Haver, M.D., Assistant Professor of Pediatrics, Harvard Medical School
Martin B. Keller, M.D., Professor of Psychiatry, Brown University
Davina Ling, Ph.D., Economist, The Medstat Group
James M. Russell, M.D., Associate Professor, University of Texas Medical Branch
Alvin Silk, Ph.D., Professor, Harvard Business School
Randall S. Stafford, M.D., Ph.D., Assistant Professor of Medicine, Harvard Medical School
Manual Trajtenberg, Ph.D., Professor of Economics, Tel Aviv University
Selected POPI Doctoral Graduates
These POPI-Supported doctoral program graduates are among those with whom POPI faculty have regular interaction, and who continue to have research interests that include economic and managerial issues related to the pharmaceutical industry:
Allan Afuah, Ph.D., 1994, Sloan School of Management; currently Associate Professor, School of Business Administration, University of Michigan
Charles King III, Ph.D., 1997, Economics; currently Assistant Professor, Graduate School of Business Administration, Harvard University
Davina Ling, Ph.D., 1999, Economics; currently an economist at The Medstat Group
Jerry Lumer, Ph.D., 1995, Economics; currently a member of the professional staff at the U.S. Department of Justice
Mark Moore, Ph.D., 1997, Economics, currently Assistant Professor, Department of Economics, University of California, Irvine
Fiona Scott Morton, Ph.D., 1994, Economics; currently Associate Professor, School of Management, Yale University
Alan Sorenson, Ph.D., Economics, 1999; currently Assistant Professor of Economics, University of California, San Diego
| Top of Page | Homepage |
POPIís success in research is a function of having a critical mass of faculty and students in the appropriate disciplines committed to conducting rigorous explorations of important issues facing the industry. These include faculty and students in scientific and clinical disciplines, as well as those in economics and management.
Research in Drug Discovery and Development
Since our inception, POPI has been researching the way research and development is organized within and among firms in the pharmaceutical industry. Our research teams have explored how the radically new environment that has emerged for the industry ó with changes in healthcare financing, rapid industry consolidation, new regulatory requirements, and significant advances in medical science and technology ó have led to major challenges and opportunities for pharmaceutical firms.
This work has led to a major new theme for our research in this area, launched in 1999 and organized under an umbrella project designated as "Science and Technology as Drivers of Change in the Pharmaceutical Industry." In this section, we provide a detailed overview of this project, and report on other projects related to drug discovery and development in which progress has been made during the past year.
Science and Technology as Drivers of Change in the Pharmaceutical Industry. The role of pharmaceuticals in healthcare has undergone dramatic change in the past ten years. During the same period, the pharmaceutical industry has seen unprecedented change, driven largely by rapidly changing science and technology. There has been a proliferation of small- to medium-size technology-based firms, new types of drugs entering therapeutic use, increasing R&D budgets at larger firms, mergers and acquisitions, and challenges in gaining and protecting intellectual property. Cross-disciplinary advances in biology, chemistry, medicine, and engineering have constituted the scientific foundation for these changes. These developments are fueling the conversion of new scientific discoveries into new drugs ó with manufacturers continuing to invest ever greater sums into R&D investments.
The competitiveness of firms in the pharmaceutical industry depends on the ability to embrace change and respond, not only with new products, but also with new operating strategies to meet the market demand for safe and efficacious drugs, especially ones that address unmet medical needs.
Past studies have addressed the relative roles and importance of industry and academia in bringing drug therapies to patients in need, the costs of bringing drugs to market, and the effect of industry structure. POPIís new project is a complement to these earlier studies, examining the effects of advancing science and technology as drivers of change in the pharmaceutical industry and how well the industry responds to the needs of the constituency it serves. Several fundamental questions inform our research: How well has the industry incorporated new science and technology into its operations for discovery, development, clinical trials, manufacturing, marketing, and distribution? Which technologies has the industry embraced? What have been the barriers to this process? Why have specific technologies been adopted and others not adopted?
Based on the presentation and discussion at a major POPI symposium held in December 1999 on the role of advancing science and technology in the evolution of the pharmaceutical industry, our research team has identified several key categories on which to focus. These include, but are not limited to:
- New approaches to drug discover (combinatorial chemistry and biology; computational chemistry; genomics)
- Methods of accelerating clinical development (imaging techniques; surrogate metrics; pharmacokinetic/dynamic solutions)
- New therapeutic strategies (gene and cellular therapies; individualized patient treatment)
- New approaches to drug delivery (transdermal; inhalation; gene therapy; polymer implants)
- New paradigms for drug distribution (e-commerce)
- Structural changes occasioned by these kinds of technologies (merger and acquisition strategies; the "virtual" pharmaceutical company that outsources discovery, development, clinical trials, manufacturing, and marketing)
The overall approach to this research revolves around the drug development paradigm, tracing specific molecules as they evolve to become drugs. With the "molecule" as the specific unit of analysis, the research team is focusing initially on Alzheimerís disease, microbial infections, asthma, cancer, and diabetes. These therapeutic areas include a mix of currently addressed indications, as well as some unmet medical needs that are currently being challenged through new drug discovery and development.
Initial efforts in this research involve the systematic identification of specific technologies that are having the greatest impact. Armed with this inventory of science and technology, the research team plans to identify firms that have embraced technological change, and which technologies have been accepted. Ongoing research will assess how well the industry incorporates new ideas, the barriers to integrating new technologies, and the lessons learned from successful technology adoption.
In POPIís view, the results from this study will enhance the knowledge of pharmaceutical firms, which will learn from our benchmarking which new scientific and technological developments have been adopted and what is being put to effective use. Regulators such as the FDA will gain insights into which technologies are ones in which regulators can have confidence, and will benefit from the identification of data that might substitute for more costly types of investigation. Through this research, patients and payors can be shown the tangible benefits to society of new scientific and technological approaches to the delivery of healthcare.
POPI faculty Stan Finkelstein, Charles Cooney, Thomas Allen, Anthony Sinskey, and Robert Rubin are conducting this research.
Balancing Incentives: The Tension Between Basic and Applied Research. This research is the work of POPI faculty Iain Cockburn, Rebecca Henderson, and Scott Stern, assisted by students Jeffrey Furman and Pierre Azoulay. Looking at for-profit pharmaceutical laboratories, the research team has tested for whether firms "balance" the provision of incentives when effort is multi-dimensional. The team finds a positive correlation between the use of promotion-based incentives for basic research and team-based incentives for applied research in drug discovery. Thus, this research provides important support in the pharmaceutical context for one of the key propositions of modern incentive theory, that is, that when a principal agent prefers agents to balance their efforts across multiple tasks, incentives will be balanced as well, and changes in incentives on one dimension will be associated with changes in incentives (in the same direction) on other dimensions.
Untangling the Origins of Competitive Advantage. This research is the work of POPI faculty Iain Cockburn, Rebecca Henderson, and Scott Stern, assisted by students Jeffrey Furman and Pierre Azoulay. In the context of the pharmaceutical industry, the team has explored the well-developed theories as to why, at any given moment, some firms (and industries) are able to earn supranormal returns, and taken those theories further to investigate the origins and dynamics of such differences in performance. They find that firms that were initially "behind" at the beginning of a given period move more rapidly to "catch up" to their more advanced competitors In addition, they find that variables traditionally associated with "strategic intent" are important determinants of the level and rate of adoption. It is clear that some firms actively identify, interpret, and act upon early signals from their internal and external environment, and so position themselves to exploit these opportunities effectively ó well in advance of othersí demonstration of the payoffs from the strategies that emerge later as "best practice." In other words, these firms are creating new sources of competitive advantage. A central question for further research is how firms organize to do so.
The Determinants of National Innovative Capacity. Under the auspices of the National Bureau of Economic Research (NBER), Scott Stern of the POPI faculty and POPI doctoral student Jeffrey Furman have collaborated with Michael Porter to conduct an empirical examination of the determinants of country-level production of international patents. They introduce a novel framework based on the concept of national innovative capacity as the ability of a country to produce and commercialize a flow of innovative technology over the long term. POPI is encouraging the investigators to examine the applicability of their work to the pharmaceutical industry.
Measuring the "Ideas" Production Function: Evidence from International Patent Output. Working with Michael Porter, and under the auspices of NBER, Scott Stern of the POPI faculty has estimated the parameters of the ideasí production function that is central to recent models of economic growth. The researchers have found that at the level of the production of international patents, country-level R&D productivity increases proportionally with the stock of ideas already discovered. Second, they find that ideas productivity in a given country is constant or declining in the worldwide stock of ideas, and that ideas production by other countries raises the bar for producing new-to-the-world technologically domestically, outweighing the positive effects of international knowledge spillovers. Finally, they find that ideas productivity is concave in the size of the R&D workforce and the linkage between ideas production and overall productivity growth is small. POPI is encouraging the investigators to examine the applicability of their work to the pharmaceutical industry.
Do Scientists Pay to Be Scientists? Scott Stern of the POPI faculty, in conjunction with NBER, has explored relationship between wages and the scientific orientation of R&D organizations. Using a sample of post-doctoral biologists, he has found suggestions of a strong negative relationship between wages and science, and that, for example, firms that allow their employees to publish extract, on average a 25% wage discount. The conclusion is that, conditional on scientific ability, scientists do indeed pay to be scientists.
Other research related to drug discovery and development. Additional POPI research in the area of drug discovery and development is being conducted by doctoral students. See the earlier section, "POPI Personnel," for descriptions of this research.
Research in the Business of Pharmaceuticals
Research into the pharmaceutical marketplace has been at the center of POPI activities since the programís inception and continued to flourish during 1999-2000. We bring together several disciplines ó economics, marketing, management science, and medicine ó to explore questions of pricing, the impact of generics, order of entry, and promotion strategies. An important area of POPI research has been our ongoing search for ways to quantify quality differences in todayís "breakthrough" drugs. And we have done pioneering work in outcomes research.
Consumption Externalities and Diffusion in Pharmaceutical Markets. Physicians and potential patient customers can gain information about the safety, efficacy and tolerability of a drug by observing the extent to which others prescribe/purchase the drug. Such consumption externality effects can operate at the level of an individual drug, or for a class of drugs having similar mechanisms of action. The POPI research team of Ernst Berndt, Robert Pindyck, and Pierre Azoulay has modeled and quantified these consumption externalities for H2-antagonists used to treat ulcers and severe heartburn, and have found evidence of their existence at the level of individual brands, but not for the overall therapeutic class.
Strategic Entry Deterrence and the Behavior of Pharmaceutical Incumbents Prior to Patent Expiration. POPI faculty Glenn Ellison and Sara Fisher Ellison have examined the behavior of pharmaceutical incumbents just prior to their loss of patent protection, looking for evidence that behavior is influenced by a desire to deter entry. Among the findings of the team are that incumbents in markets of intermediate size are more likely to reduce detail advertising and to increase the proliferation of presentations immediately prior to patent expiration.
Medical Care Prices and Output. POPI faculty member Ernst Berndt and the late Zvi Griliches, in conjunction with other researchers, have explored the difficult issues raised by measurement of medical care prices and the output of the medical care service industries. Surveying and summarizing the underlying conceptual issues and discussing in considerable detail how the official Bureau of Labor Statistics Producer and Consumer Price Index programmers deal with these measurement issues, the research team has developed several recommendations that would improve and augment the official price and output statistics.
The Medical Treatment of Depression. POPI faculty member Ernst Berndt, working with a team at NBER, integrated retrospective medical claims data involving the treatment of acute phase major depressive disorders with expert panel evaluations on the likely outcomes of alternative psychotherapy/pharmacotherapy treatment modalities. The researchers then constructed aggregate price indices for the treatment of depression, taking into account changing treatment choices over time, expected medical outcomes from these treatments, and variations over time in the patient severity mix. The team finds that over the 1991-96 period the cost of obtaining an expected symptomatic remission from an episode of antidepressant treatment declined about 2.6% per year.
Healthcare Use and At-Work Productivity Among Employers with Mental Disorders. POPI faculty Ernst Berndt and Stan Finkelstein, along with student Jason Verner and others, have examined the extent to which employees at a major national data processing firm are treated for anxiety and other mental disorders, and whether there are any average daily productivity differences among those treated for anxiety only, anxiety plus another comorbid mental disorder and those not receiving any treatment for a mental disorder. The research team has found no significant average daily productivity differences among these groups. Medical care utilization is lowest for those receiving no treatment for any mental disorder, is higher for those receiving treatment for only one mental disorder, and is highest for those having several mental disorder comorbidities.
Comparing SSRI Treatment Costs for Depression Using Retrospective Claims Data. Using retrospective medical claims from MedStat, Ernst Berndt and others have examined how cost-effectiveness comparisons among three selective serotonin reuptake inhibitors are affected when alternative statistical methods are employed to deal with skewed data sample selectivity, and outlier observations. The data cover a period by which all three SSRIs had extensive histories concerning efficacy, side effects ,and tolerability.
Lost Human Capital from Early-Onset Chronic Depression. In a study with significant public policy implications, Ernst Berndt, Stan Finkelstein, and others have integrated data from a randomized double-blind clinical trial with U.S. Census Bureau data on 1995 earnings by age, educational attainment, and gender, finding that early-onset major depressive disorder adversely affected the educational attainment of women but not of men. No significant difference in treatment responsiveness by age at onset was observed after 12 weeks of acute treatment or, for subjects rated as having responded, after 76 weeks of maintenance treatment. A randomly selected 21-year-old woman with early-onset major depressive disorder in 1995 could expect future annual earnings that were 12%-18% lower than those of a randomly selected 21-year-old woman whose onset of major depressive disorder occurred after age 21 or not at all.
The Diffusion of New Antidepressant Medications. With several other researchers, POPI faculty member Ernst Berndt has examined empirically the impacts of product attributes, variety in these attributes, marketing efforts, order of entry, and pricing on the diffusion of new pharmaceutical medications, using drugs in the therapeutic class for treatment of major depressive disorder. The team has quantified the impacts of these various factors on the overall market for antidepressants, as well as on sales of individual molecules.
The U.S. Pharmaceutical Industry: Why Significant Growth in Times of Lost Containment? POPI faculty member Ernst Berndt has examined the growth in utilization (rather than growth in prices) that has been the primary driver of increased pharmaceutical expenditures in the last five years. The study explored four factors that help explain why the utilization of pharmaceuticals has continued to grow even as managed care and other cost-containment efforts have flourished: "the importance of being unimportant"; the increase in third-party prescription drug insurance coverage; successful new product innovations; and aggressive technology transfer and marketing efforts.
Pharmaceutical Prices and Political Activity. POPI faculty member Sara Fisher Ellison, working with Catherine Wolfram of Harvard University, has studied the possible effects of political activity on pharmaceutical prices, with a particular focus on the period of the healthcare reform debates of 1993-1994 in the United States. The researchers evaluate the extent to which pharmaceutical companies slowed the rates at which they increased prices in an attempt to preempt government interventions. The results of the study suggest that companies whose drugs had longer patent lives and who had recently increased contributions to their corporate political action committees (PACs) slowed prices during the period in question more than their competitors.
Gradual Incorporation of Information: Pharmaceutical Stocks and the Evolution of Clintonís Healthcare Reform. Working with Wallace Mullin of Michigan State University, POPI faculty member Sara Fisher Ellison examined how pharmaceutical stock prices reacted to the Clinton healthcare reform efforts from January 1992 to October 1993. Noting a 52.3% decline in market-adjusted pharmaceutical prices, the researchers associate this change with the healthcare reform. They then find indirect evidence to suggest that the wealth lost by pharmaceutical firms may have been largely an anticipated transfer rather than a social welfare loss. The findings are relevant to the understanding of the likely effects of healthcare reform plans that may contain elements similar to the Clinton plan. Whereas many other public policy changes are marked by a process of gradual public revelation of information (which may not be completely observable by a researcher), the teamís approach may uncover information about the anticipated effects of policies not available from a traditional event study.
The Impact of Benefit Plan Design on Prescription Drug Utilization and Health Outcomes. Working with William Crown of The MEDSTAT Group, Inc., POPI faculty Ernst Berndt and Stan Finkelstein are contributing to the debate among policymakers, health insurance companies, managed care plans, and other stakeholders regarding prescription drug coverage and its effects on utilization and outcomes. Using asthma as a case study ó with the rising prevalence of asthma among adults and children as well as the high economic costs associated with asthma ó the team is working to quantify the impact of drug coverage characteristics on the utilization of asthma prescription drugs, based on the MarketScanģ Databases of privately insured healthcare. The researchers are developing descriptive analyses that will be used to estimate multivariate models. The results will assist the pharmaceutical industry in predicting the impact of the generosity of prescription drug coverage on clinical and economic outcomes. This project has been encouraged by the pharmaceutical industryís Ad Hoc Group on Economics.
Asthma Care and Outcomes Among Public and Private Insured. Related to the research above, Finkelstein, Berndt, and Crown are assessing empirically the determinants of asthma prevalence, treatment selection, and outcomes using some of the largest retrospective claims datasets available from employers and state Medicaid programs. The overall aim of the study is to provide insights for designing prospective interventions, and ultimately public policies and clinical practice, the main goals of which will be to reduce the public health burden of asthma.
Time-Series Analysis of Factors Influencing Medication Prescribing. POPI faculty Stan Finkelstein and Iain Cockburn, in collaboration with Randall Stafford, M.D. of Massachusetts General Hospital, are developing a model of the simultaneous influence of multiple determinants on trends in medication prescribing. With the model, the researchers will assess the relative influence of clinical guidelines, clinical trial results, pharmaceutical promotion, drug prices, and specific competitive features of the pharmaceutical market. The results will be relevant to efforts to design and implement clinical and health policies to encourage the effective and cost-effective use of medications
Selected New Articles and Working Papers, 1999-2000
Berndt, Ernst R., "The U.S. Pharmaceutical Industry: Why Significant Growth in Times of Lost Containment?", Health Affairs, forthcoming 2001.
Berndt, Ernst R., Davina Ling, Margaret M. Kyle and Stan N. Finkelstein, "The Long Shadow of Patent Expiration: Do Rx to OTC Switches Provide an Affiliate?", September 2000.
Berndt, Ernst R., Howard Bailit, Martin B. Keller, Jason C. Verner and Stan N. Finkelstein, "Heath Care Use and At-Work Productivity Among Employees with Mental Disorders", Health Affairs, 19(2), pp. 224-256, July/August 2000.
Berndt, Ernst R., Anupa Bir, Susan Busch, Richard G. Frank and Sharon Lise-Normand, "The Medical Treatment of Depression, 1991-1996: Productive Inefficiency, Expected Outcome Variations, and Price Indexes", July 2000.
Berndt, Ernst R., Ashoke Bhattacharjya, David N. Mishol, Almudena Arcelus, and Thomas Lasky, "Variety, Evaluation, Order of Entry and Marketing Efforts: An Analysis of the Diffusion of New Antidepressant Medications", Submitted for publication to a peer-reviewed journal, June 2000.
Berndt, Ernst R., Lorrin M. Koran, Stan N. Finkelstein, Alan J. Gelenberg, Susan G. Kornstein, Ivan M. Miller, Michael E. Thase and Martin B. Keller, "Lost Human Capital from Early-Onset Chronic Depression", American Journal of Psychiatry, 15726, pp. 940-947, June 2000.
Berndt, Ernst R., Robert S. Pindyck and Pierre Azoulay, "Consumption Externalities and Diffusion in Pharmaceutical Markets: Anti-Anti-ulcer Drugs", June 2000.
Berndt, Ernst R., Salvatore N. Colucci, Amy N. Grudzinski, Robert Micel, James M. Russell and Yikang Xu, "Comparing SSRI Treatment Costs for Depression Using Retrospective Claims Data: The Role of Nomrandom Selection and Skewed Data", Value in Health (Official publication of the International Society of Pharmacoeconomics and Outcomes Research), Vol. 3, No. 3, pp. 208-227, May/June 2000.
Berndt, Ernst R., "International Comparisons of Pharmaceutical Prices: What Do We Know, and What Does It Mean?", Journal of Health Economics, 19:2, pp. 283-287, April 2000.
Berndt, Ernst R., David M. Cutler, Richard G. Frank, Zvi Griliches, Joseph P. Newhouse and Jack E. Triplett, in Anthony C. Culyer and Joseph P. Newhouse, editor, Medical Care Prices and Output, Handbook of Health Economics, Amersterdam: Elsevier Science, Vol. 1A, pp. 120-130, 2000.
Cockburn, Iain M., Rebecca Henderson, and Scott Stern, "Disentangling the Origins of Competitive Advantage", Strategic Management Journal, forthcoming.
Cockburn, Iain M., Rebecca Henderson and Scott Stern, "Balancing Incentives: The Tension Between Basic and Applied Research", NBER WP 6882, submitted to American Economic Review, July 2000.
Cockburn, Iain M., Rebecca M. Henderson and Scott Stern, "Untangling the Origins of Competitive Advantage", Sloan WP #4109, March 2000.
Cockburn, Iain M., Rebecca Henderson and Scott Stern, "The Diffusion of Science-Driven Drug Discovery: Organizational Change in Pharmaceutical Research", NBER WP 7359, under revision for resubmission to Journal of Economics, Management and Strategy, September 1999.
Ellickson, Paul, Scott Stern and Manuel Trajtenberg, "Patient Welfare and Patient Compliance: An Empirical Framework for Measuring the Benefits Of Pharmaceutical Innovation", Medical Care Output & Productivity, NBER/CRIW Conference volume, forthcoming, and NBER Working Paper 6890, January 1999.
Ellison, Glenn, and Sara F. Ellison, "Strategic Entry Deterrence and the Behavior of Pharmaceutical Incumbents Prior to Patent Expiration", submitted to Econometricia, 2000.
Ellison, Sara F., and Wallace Mullin, "Gradual Incorporation of Information: Pharmaceutical Stocks and the Evolution of Healthcare Reform", returned for revision by The Journal of Law and Economics, 2000.
Ellison, Sara F., and Catherine Wolfram, "Pharmaceutical Prices and Political Activity", submitted to Quarterly Journal of Economics, 2000.
Ellison, Sara F., and Judy Hellerstein, "The Economics of Antibiotics", Measuring the Prices of Medical Treatments, AEI-Brookings Conference Volume 1999.
Healy, Paul M., Stewart C. Myers and Christopher D. Howe, "R&D Accounting And The Tradeoff Between Relevance and Objectivity", January 1999.
King, Charles, III, Alvin J. Silk, Lisa R. Klein and Ernst R. Berndt, Harvard Business School, Case No. N9-500-073, 15 pp., February 4, 2000.
Stern, Scott, "Do Scientists Pay to be Scientists?", NBER WP 7410, submitted to Journal of Political Economy, 2000.
Stern, Scott, and Manuel Trajtenberg, "Empirical Implications of Physician Authority in Pharmaceutical Decisionmaking", NBER WP 6851, under revision for resubmission to Journal of Health Economics, 2000.
Stern, Scott, "Market Definition and the Returns to Innovation: Substitution Patterns in Pharmaceutical Markets", MIT POPI Working Paper #36-96, December, 1996, under revision for first submission to Review of Economics and Statistics.
| Top of Page | Homepage |
POPI faculty are currently involved in developing two new, related degree programs. We are planning to offer a masterís degree in management of technology specifically to persons with experience in the biomedical industries. This program will draw upon the Sloan Schoolís management of technology program, now entering its third decade. This one-year program is geared toward mid-career executives. In addition, we plan to offer a dual-degree program, the Biomedical Enterprise Program, in which students will receive a masterís in the management of technology from the Sloan School and a masterís degree from the Harvard-MIT Division of Health Sciences and Technology.
Since our founding in 1991, POPI has offered a number of subjects as part of MITís regular academic catalogue, and have participated in, and thus strengthened, courses that predate POPI. We also offer a successful on-campus summer executive program.
Course offerings have included subjects on pharmaceutical and biotechnology industry management, health technology, comparative health systems, and industrial economics for strategic decision-making. Selected recent developments include the following:
- Principles and Practices of Drug Development . This MIT subject, offered jointly in the MIT departments of biology and chemical engineering, the MIT Sloan School of Management, and the Harvard-MIT Division of Health Sciences and Technology, attracted an enrollment of nearly 50 students from across MIT, and included presentations by regular faculty and industry guests.
- Medical Outcomes Assessment and Pharmacoeconomics . This MIT subject offered in the Sloan School of Management had an enrollment of nearly 30 students, and included presentations by regular faculty and industry guests.
- Science and Technology Driving Change in the Drug Discovery, Development, and the Business of Pharmaceuticals . This December 1999 POPI Symposium on the Future of the Pharmaceutical Industry was attended by 250 people, including many executives from the pharmaceutical industry. The symposium was followed by a June 2000 weeklong Special Summer Program with 20 enrollees from industry, academia, and government.
- Pepcid AC: Racing to Enter the OTC Market . This Harvard Business School case study (N9-500-073) by Charles King III, Alvin J. Silk, Lisa R. Klein, and Ernst R. Berndt examines the strategies and results as the manufacturers of four patent-protected H2-antagonist prescription-only drugs raced to obtain approval from the U.S. Food & Drug Administration to market over-the-counter versions, focusing on alternative ways of researching the market and on the construction of clinical trials.
| Top of Page | Homepage |
POPI has influenced public policy debates in a wide variety of ways. POPI personnel have interacted with officials at the U.S. Department of Labor, the U.S. Department of the Treasury, and the Presidentís Council of Economic Advisors, to name a few. Other POPI faculty have been closely involved with the U.S. Food & Drug Administration and have influenced important changes in the internal procedures of the agency. While our participation is not always highly visible, POPI is quite influential.
More visibly, POPI faculty over the years become regular participants on policy panels related to the pharmaceutical industry and healthcare. Our researchers have also brought insights from their research to testimony before the U.S. Congress and other legislative and regulatory bodies.
POPI is committed to the dissemination of our research findings to the broader business and public policy communities. We strongly encourage our faculty to search out opportunities to present their research and to apply their expertise to relevant policy and business issues.
Major Ongoing Activities
Two different projects, involving six POPI faculty from across MIT, are specifically addressing the public policy arena.
- Science and Technology Driving Change in Drug Discovery, Development, and the Business of Pharmaceuticals
In December 1999, POPI held its biennial symposium on "The Future of the Pharmaceutical Industry." Approximately 250 executives from pharmaceutical companies and government agencies attended. At this symposium, POPI launched this major initiative around the theme of the gathering: "Science and Technology Driving Change in Drug Discovery, Development, and the Business of Pharmaceuticals." In addition to the research and educational programs underway (described earlier in this report), plans are underway to prepare a monograph and for faculty to be available to present the findings in the public policy arena. POPIís monograph will focus, in part, on the returns to investment in pharmaceutical R&D that has assimilated key scientific and technological advances in drug discovery and development.
Five faculty are participating in this effort: Thomas Allen, Ph.D. (Management of Technology); Charles Cooney, Ph.D. (Chemical Engineering); Stan Finkelstein, M.D. (Health Economics); Robert Rubin, M.D. (Clinical Pharmacology); and Anthony Sinskey, Ph.D. (Biological Sciences).
In the spring of 2000, POPI received a grant from PhRMA to continue activities in this area.
- Prescription Drug Benefit for Residents of Massachusetts
Early in 1999, POPI was asked by the co-chairs of the Health Care Committee of the Massachusetts Legislature to facilitate a discussion between the pharmaceutical industry and government officials on the topic of a prescription drug benefit for senior citizens and certain other residents of Massachusetts. An informal, off-the-record discussion was held at MIT in June 1999, with representatives of PhRMA, five pharmaceutical firms, two members of the Massachusetts Legislature and their staffs, the president of Massachusetts College of Pharmacy, and two POPI faculty, Ernst Berndt and Stan Finkelstein.
Subsequent to this discussion, Drs. Berndt and Finkelstein have become involved in the evaluation of two pieces of legislation that were recently enacted by the Massachusetts Legislature. The first provides for volume purchasing of pharmaceuticals for constituencies covered by state-supported insurance programs. The other, based on a recent proposal by the Heinz Foundation, would establish a subscriber-based prescription drug benefit for senior citizens. POPI faculty members look forward to completing this evaluation and to preparing a report to be disseminated to relevant policy officials.
Other Activities and Accomplishments
- POPI faculty member Ernst Berndt has been active in helping to interpret recent trends in prices of pharmaceuticals and growth in sales volume. He presented an analysis of these trends at a White House/DHHS Conference held in August 2000. In October 2000, he will brief the 1 board of directors on this topic. Earlier, he discussed these ideas with a group of distinguished journalists who are currently Kaiser Family Foundation Media Fellows. Professor Berndt also prepared a review of the work of Professor Patricia Danzon (The Wharton School) on cross-national comparisons of pharmaceutical prices, which was published as commentary in Journal of Health Economics.
- Stan Finkelstein participated in a conference organized by the National Institute of Disease of Digestion and the Kidney of the National Institutes of Health that was directed at an important issue of clinical policy. The meeting, held in September 2000, was called to determine whether there is sufficient knowledge and interest to organize a therapeutic development program to address acute renal failure.
- POPI research conducted by Rebecca Henderson and Iain Cockburn has been quoted prominently in The New York Times. While we believe that some of the material reported in the media and attributed to POPI was not correctly interpreted in the article, the media publicity stimulated numerous requests for their articles and working papers and provided a number of opportunities for these faculty to interact with public policy officials and reporters.
- Stan Finkelstein and Anthony Sinskey conducted a briefing for Kaiser Family Foundation Media Fellows (a group of distinguished journalists) in August 2000 on the new POPI initiative on "Science and Technology Driving Change in Drug Discovery, Development, and the Business of Pharmaceuticals."
- Several POPI faculty members, including Ernst Berndt, Rebecca Henderson, and Iain Cockburn, participate frequently in conferences organized by the Brookings Institute, the American Enterprise Institute, and the National Academy of Sciences, all Washington-based policy-research organizations. The topics have related to technological innovation, R&D policy, and prescription drug policy.
| Top of Page | Homepage |