
The first X-ray spectrometer. [Science Museum London]
In the popular imagination, the New Physics which conquered the intellectual world between 1890 and 1950 is all of a piece. Radioactivity, relativity (special and general), quantum mechanics, the "particle zoo" -- today all, with the notorious exception of gravity, seem well-integrated parts of the Standard-Model machine, and it is difficult to imagine that the pioneers of one ignored (or occasionally opposed) the rest. Such however was often the case.
Those heroes of early modern-physics who objected publicly to its later development (Einstein the obvious example) at least fit the story of Progress triumphing over conservative opposition even from unexpected quarters, while such villainous characters as Johannes Stark seem more natural in the reactionary role than in their earlier manifestations.
More common than resistance, however, were ignorance and indifference. Although scientists of the early 1900s were by no means so narrowly specialised as their descendants, they had somewhat smaller fields of interest than their predecessors, and they neglected (or sometimes chose to neglect) developments outside of their main area. Even within a subdiscipline there could be surprising failures of internal communication, often on national lines. Most British, for instance, did not so much reject Einstein's work as pay no attention to it (at least until well after the end of the Great War). Quantum mechanics (that is, the "old" quantum theory of Bohr and Sommerfeld) was better known, but for all of Cambridge's excellence in applied mathematics, the British at first contributed to the revolution mainly as experimentalists, and preferred a readily-visualised mechanics of tiny solar-systems or plum-puddings to German philosophy's unimaginable abstractions.
These national traits can be seen in this week's feature, a speech delivered by one of the British Empire's greatest physicists.
Despite his jarring references to "ether" and his merely passing (though highly favourable) mention of Niels Bohr, Bragg shows himself here as one of the best popularisers of the (emerging) new field of experimental microphysics, in which he and his son will always be numbered among the Titans. Listening to him speak (almost on the eve of the Heisenberg-Schrödinger synthesis) about the confusing nature of the quantum world and the oddly practical results obtainable in the midst of the confusion reminds one of how exciting was the life of a physicist during the change of paradigms --- and it is perhaps worth mentioning that some of the mysteries he cites have never been resolved.

ELECTRONS AND ETHER WAVES
by Sir William Henry Bragg, F. R. S.
The Robert Boyle Lecture at Oxford University for the year 1921
Sir William's words are in bold.
I propose to ask you this evening to consider for a short time one of the outstanding problems in physics. I am justified, I think, in saying that so far it has proved insoluble, but for all that, it lacks neither interest nor importance. It is important because it relates to very fundamental things with which we are deeply concerned, and as to its interest, it comes in many ways.
Man's interest in radiation is naturally very old indeed. The warmth of the sun, the light that it gives by day, and the light of the moon and stars by night, fill a first place in their importance to him. When experimental science began to grow rapidly, its first efforts were devoted to an attempt to unravel the laws of propagation of light and heat. Among the famous pioneers Newton and Huyghens represented two opposing schools of thought. The former advocated a corpuscular theory of light, the latter maintained that light consisted of a wave motion.
In a restricted sense, the wave theory has completely triumphed; it explains the ordinary phenomena of light and especially of the intricate effects which depend on interference of waves with the greatest satisfaction and precision. But, on a wider view of light phenomena, the victory of the wave theory is not so absolute, for it is certain that a great part is played by corpuscular radiations, the corpuscles being the electrons of recent discovery. It seems that we must admit the importance of each view and, to a certain extent, we can accurately define the part that each must play: but, there is one great exception. There is one problem in connection with the interrelations of electron waves and corpuscles which seems to ridicule all our attempts to understand it. If we could solve it we should have made an immense advance, both in knowledge and in our power of handling materials. We should perhaps have added a new province to the realms of physical thought.
And it is because of this obvious importance and because of our failures to find the solution that I hope you will be interested in looking at the question once again in the light of recently acquired knowledge.
We are going to consider the relations between the energies carried by ether waves and the energy carried by electrons. Let us first set down the distinctive features of each form of radiation.
As regards wave radiation, we must say that the energy spreads outwards and weakens as it spreads, just as a sound dies away in the open air. And next we must add that all waves show the extraordinary phenomenon of interference. Two sets of waves can tend to destroy each other's actions at certain places and times, making good such losses by increased actions at other places and other times. By the aid of this principle, Young and Fresnel, and a host of workers who have followed them, have built up optical theories of great power and completeness. Note that the characteristics of a simple wave are its length and its amplitude: it has no others.

Figure by Kurzon
Corpuscular radiations have been obvious to us on the grand scale only since the discovery of radium and of X-rays. Beside the X-rays, the projection of helium atoms from the bursting atoms of radio-active substances, we find in the general radiation of radio-active substances streams of high speed electrons. The main features of these rays which concern us now can also be stated briefly :
Electrons are to be found everywhere forming part of every atom. They can be set in motion by electric forces, as in the X-ray tube, or they may be expelled from radio-active substances. Such radiation like light radiation has qualities. The flying particles may be more or less in number, and the speed of each can fall between wide limits. In other respects it is, at present, assumed that they are all like each other.
We have not been acquainted with electron movements so long as we have been acquainted with wave motions in ether. The reason is perhaps a simple one:
An electron can only maintain a separate existence if it is traveling at an immense rate : from one three-hundredth of the velocity of light upwards, that is to say, at least 600 miles a second or thereabouts. Otherwise the electron sticks to the first atom it meets. The action of a powerful induction coil and space to move in freely, where there are no atoms to impede it, provide favorable circumstances for observation, and we have only been able to realize these conditions with sufficient success in more recent years.
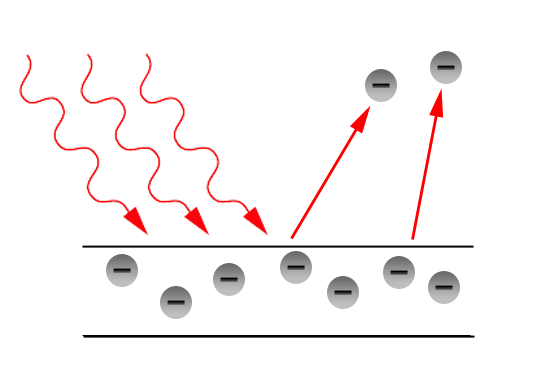
Figure by Feitscherg
We now know, therefore, radiation in two forms, and each is independently full of interest. But it is the extraordinary connection between them that is so fascinating and yet beats us when we try to explain it. We have known for many years that there is some connection between waves and electrons because light, especially of short wave length, can cause a discharge of negative electricity, that is to say, of electrons, from substances on which it falls. This, which is known as the photo-electric effect, has been carefully examined with a view to discovering relations between the wave length of the ether radiations and the velocity of the ejected electrons. But the experimental difficulties of obtaining a close insight into the effect were always considerable until we had to do with the new variety of light which Röntgen discovered. The very short wave length which is associated with X-rays goes with a photo-electric effect which is so greatly intensified that we can examine it in detail, and now the relation between wave and electron takes on an importance which arrests attention.
We can take the question in two stages : in the first as a general question. In the second we bring in effects which depend on details of atomic structure.
The general question can be stated quite simply. We have seen that a wave motion is defined by two qualities. The one, the wave length; the other the amplitude. When an X-ray falls upon any material substance we find that electrons are ejected: the wave radiation has produced an electron radiation. Electron radiation has characteristics also, namely, number and speed. In what way then are the characteristics of the waves related to the characteristics of the electron movements which are excited by them? The answer is simple but surely unexpected.
The velocity of the electron depends on the wave length only ; the number of electrons depends on the intensity, but not on the wave length. Moreover, the relation between the wave length of the one radiation and the velocity of the other is of the simplest kind. If we define the wave length by stating the number of waves that pass by a given point in a second and call this number the frequency, then the energy of the electron is equal to the frequency multiplied by a constant quantity. This constant is not new to us, it had already turned up in connection with investigation of interchange of energy, where waves are concerned, and is well known as Planck's constant. That, however, need not concern us now.
The essential point is that a wave radiation falling on matter of any kind whatever and in any physical condition, liquid or solid or gaseous, hot or cold, causes the ejection of electrons. In actual experiment we cannot usually examine the speed of the electron at the instant of its production. We have generally to wait for the electrons to get outside the body in which they arise before we can handle them in our experiments. Those that have come through the deeps of the material have lost speed by collision with the atoms on their way out. Consequently, we have in response to the incidence of waves of a definite frequency, that is to say, of so-called monochromatic radiation, an output of electrons of various speeds ranging downwards from a maximum which is given by the above-mentioned relation. There does not seem to be any doubt that the electrons all had originally quite the same definite speed, and that the differences in speed are acquired subsequently.
In this process we see energy of wave radiation replaced by energy of electron radiation. There is an exactly converse process. If we direct a stream of electrons against any material substance, we can call into being ether waves. They arise at the point of impact and their quality is, in the general sense, determined by the velocity which we have given the electron stream.
Among the waves so originated there are some whose frequency is related to the energy of the individual electron in the electron stream by the same constant as before. There are others of lesser frequency, such as we might suppose to be originated by electrons that belonged to the original stream, but have lost energy by collisions with the atoms of matter. Here again, there is no doubt that the electrons produce waves for which the frequency is exactly determined by the use of Planck's constant as above.

Photo by Jon Sullivan
In order to realize the full significance of these extraordinary results, let us picture the double process as it occurs whenever we use an X-ray bulb. By the imposition of great electrical forces we hurl electrons in a stream across the bulb. One of these electrons, let us say, starts a wave where it falls. This action is quite unaffected by the presence of similar actions in the neighborhood, so that we can fix our minds upon this one electron, and the wave which it alone causes to arise. The wave spreads away, it passes through the walls of the bulb, through the air outside, and some where or other in its path in one of the many atoms it passes over an electron springs into existence, having the same speed as the original electron in the X-ray bulb. The equality of the two speeds is not necessary to the significance of this extraordinary effect ; it would have been just as wonderful if one speed had only been one half or one quarter or any reasonable fraction of the other. The equality is more an indication to us of how to look for an explanation than an additional difficulty to be overcome.
Let me take an analogy. I drop a log of wood into the sea from a height, let us say, of 100 feet. A wave radiates away from where it falls. Here is the corpuscular radiation producing a wave. The wave spreads, its energy is more and more widely distributed, the ripples get less and less in height. At a short distance, a few hundred yards perhaps, the effect will apparently have disappeared. If the water were perfectly free from viscosity and there were no other causes to fritter away the energy of the waves, they would travel, let us say, 1,000 miles. By which time the height of the ripples would be, as we can readily imagine, extremely small.
Then, at some one point on its circumference, the ripple encounters a wooden ship. It may have encountered thousands of ships before that and nothing has happened, but in this one particular case the unexpected happens. One of the ship's timbers suddenly flies up in the air to exactly 100 feet, that is to say, if it got clear away from the ship without having to crash through parts of the rigging or something else of the structure. The problem is, where did the energy come from that shot this plank into the air, and why was its velocity so exactly related to that of the plank which was dropped into the water 1,000 miles away? It is this problem that leaves us guessing.
Shall we suppose that there was an explosive charge in the ship ready to go off, and that the ripple pulled the trigger? If we take this line of explanation we have to arrange in some way that there are explosive charges of all varieties of strength, each one ready to go off when the right ripple comes along. The right ripple, it is to be remembered, is the one whose frequency multiplied by the constant factor is equal to the energy set free by the explosion. The ship carries about all these charges at all times, or at least there are a large number of ships each of which carries some of the charges, and externally the ships are exactly alike.
Also we have to explain why, if we may drop our analogy and come back to the real thing, the ejected electron tends to start its career in the direction from which the wave came. This is a very marked effect when the waves are very short.
Dropping the analogy, how do the electrons acquire their energy and their direction of movement from waves whose energy and momentum have become infinitesimally small at the spot where they are affected, unless the atom has a mechanism of the most complicated kind ? And if the intervention of the atom is so important, why is it that in these effects a consequence of the intervention does not depend upon each atom itself --- whether, for example, it is oxygen or copper or lead?

Henry Moseley's famous "step ladder".
We may try another line of explanation and suppose that the energy is actually transferred by the wave from the one electron to the other. If it is the atom which pulls the trigger and causes the transformation, then how does it happen that the whole of the energy collected by the wave at its origin can be delivered at one spot? Rayleigh has told us that an electron over which a wave is passing can collect the energy from an area round about it whose linear dimensions are of the order of the wave length. But any explanation of this kind is entirely inadequate. Whatever process goes on, it is powerful enough on occasion to transfer the whole of the energy of the one electron to the other.
Nor can there be any question of storing up energy for a long period of time until sufficient is acquired for the explosion. For it is not difficult to show that when an X-ray bulb is started and its rays radiate out, the actual amount of energy which can be picked up by an atom a few feet away would not be sufficient for the ejected electron, though the tube were running for months; whereas we find the result to be instantaneous.
I think it is fair to say that in all optical questions concerned with the general distribution of energy from a radiating source the wave theory is clearly a full explanation. It is only when we come to consider the movements of the electrons which both cause waves and are caused by them that we find ourselves at a loss for an explanation. The effects are as if the energy were conveyed from place to place in entities, such as Newton's old corpuscular theory of light provides. This is the problem for which no satisfactory solution has been provided as yet : that at least is how it seems to me.

The arms chosen by Niels Bohr. [Figure by G. Jo]
No known theory can be distorted so as to provide even an approximate explanation. There must be some fact of which we are entirely ignorant and whose discovery may revolutionize our views of the relations between waves and ether and matter. For the present we have to work on both theories. On Mondays, Wednesdays, and Fridays we use the wave theory; on Tuesdays, Thursdays, and Saturdays we think in streams of flying energy quanta or corpuscles!
That is after all a very proper attitude to take. We cannot state the whole truth since we have only partial statements, each covering a portion of the field. When we want to work in any one portion of the field or other, we must take out the right map. Some day we shall piece all the maps together.
Meanwhile, even if we cannot explain the phenomena we must accept their existence and take account of them in our investigations. We must recognize that wave radiation and electron radiation are in a sense mutually convertible. Whenever there is one there must be the other, provided only there is matter to do the transforming. We do not yet know more than a little of the part that this process of interchange plays, but we know that it is very prominent when the waves are very short, or, what is the same thing, the electrons moving swiftly. It is the movement of the electrons in the X-ray bulb that originates the X-rays themselves. They as waves pass easily through the wall in the tube and through materials outside: their energy finally disappears and is replaced by moving electrons. It is the latter alone that produce directly the effects which we ascribe to X-rays. We may suspect that similar effects to these take place when the waves are long, but the corresponding electron velocities are so small that it is difficult to measure them or observe their effects. Nevertheless, the carrying-forward to these regions of experience gained elsewhere has led to extraordinary results, as for example, in the theories of Bohr regarding the relations between the structure of an atom and the radiation it emits.
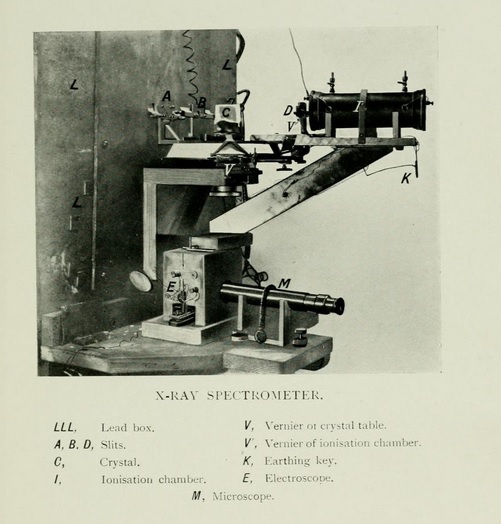
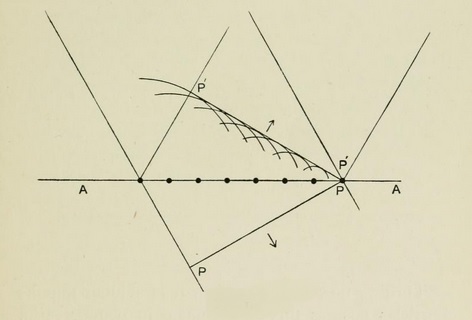
Illustration from Bragg's X Rays and Crystal Structure [London: Bell, 1915]
I have spoken of the first stage in this examination of the relations between ether waves and electrons. May I now go one step further and bring in certain curious and lately discovered relations between the interchanges and the nature of the atom itself? All that I have said before is mainly independent of atomic nature ; I want now to consider certain experimental results which are superimposed upon the former without in the least invalidating them and which obviously have a first importance on our appreciation of atomic structure.
When an X-ray of given wave length strikes an atom, it may result in the ejection of an electron of equivalent energy as described above. And in such a relation between wave length and energy there can be no trace of any influence of the nature of the atom. But it may sometimes happen that the energy instead of being handed over or transformed in one complete whole is transformed in a series of successive stages, and these stages are really characteristic of the atom. Let me give an illustration :
Let us imagine an X-ray of wave length equal to two-tenths of an Ångstrom unit (100-millionth of a centimeter), such as comes, under ordinary circumstances, from a powerful X-ray bulb. It falls on a silver atom ; it may, as in the general process, produce an electron of energy equivalent to itself, but it may also divide up this energy into two parts. One part is characteristic of the silver atom. It is an amount which the silver atom is for some reason especially liable to absorb or develop. It is peculiar to the silver atom, no other atom absorbs just that quantity.

X-ray spectrum of rhodium, with two characteristic K lines superimposed on the universal, continuous Bremsstrahlung spectrum, and Duane-Hunt cutoff at wavelength inversely proportional to the voltage of the X-ray tube, in this case 60kV. [Wikipedia; figure by "Linguistic Demographer".]
Leaving out of account for the moment the balance, let us follow the course of happenings to this particular quantity of energy. It excites in the atom a series of rays characteristic of the atom. These rays are divided into groups characteristic of the atom, but of a general arrangement which is the same for all atoms. It appears that the absorbed energy is divided up between various rays, probably giving rise to one out of each group, and in that way its whole total is spent.
These rays we now analyse with an X-ray spectrometer using a crystal as our diffraction grating. It is by their use that we have been able to study the architecture of crystals and to find the way in which the atoms, under the influence of their mutual force, arrange themselves in crystalline form.
Going back for a moment to the balance, the difference between the energy characteristic of the original X-ray and that amount of energy which was used up in the way just described, this energy, it appears, is found in the possession of an electron whose velocity can be measured with accuracy. Here we have an extraordinary instance of a partition of energy between wave and electron. We find the action of a wave resulting in the initiation of both electrons and waves, but the simple relation which we had in the general case is only modified to a slight degree. There may be several items instead of one in our balance sheet, but the balance is still good. This action follows just as well as a consequence of the impact of an electron having the necessary energy as it does from the incidence of an X-ray in the way I have described.
We should notice in addition that when X-rays or electrons fall short in their associated energy of the amount characteristic of the atoms, there is no result at all, and this is reflected in the fact that neither of them is absorbed in the atom so much as if they were respectively a little higher in frequency or a little greater in velocity.
The curious and essential feature of all this mass of information which I have been trying to put before you in a rough and summary form is the interchangeability of ether waves and electrons. Energy can be transferred from one to another through the agency of matter. The transference is governed by the simplest arithmetical rules. In the exchange it is the frequency of the wave which is to be set against the energy of the electron, and it is just this that makes the greatest puzzle in modern physics. It is the block at one point which is choking the entire traffic and on which, therefore, all our interests must concentrate.

Figure by B. V. Crist