
Understanding self-organisation -- the apparently spontaneous emergence of complex order from random chaos -- is perhaps the greatest challenge faced by Twenty-First Century physics. Of course, the problem is in no sense a new one: in the last century different aspects of it were tackled by the whole tribe of chaos theorists, by Ilya Prigogine, by Claude Shannon, by Alan Turing, even by Sir Fred Hoyle. In the century before that, Poincaré laid the foundations of non-linear dynamics, the heroic generation of thermodynamicists struggled to formulate the concept of entropy, and Charles Darwin proposed a mechanism by which at least living beings, if not inanimate ones, could evolve from primordial simplicity.
A December 2015 e-print by developmental biologist Raphaël Clément of Aix-Marseille Université draws attention to a forgotten -- and when not forgotten much-ridiculed -- pioneer of the field. Stéphane Leduc was a medical doctor and an experimental biophysicist before biophysics in the Twentieth Century sense existed. In the years between 1900 and the Great War (the years of relativity and the old quantum theory) he proposed a radically materialistic approach to the question of life. "The physics of vital action," he wrote, "are the physics of the phenomena which occur in liquids, and the study of the physics of a liquid must be the preface and the basis of all inquiry into the nature and origin of life."
Specifically, Leduc believed that hydrodynamic processes, diffusion, and osmosis are responsible for most of biology. To illustrate this, he conducted extraordinary experiments with liquids, often far from equilibrium. Although relying on intuition and observation rather than higher mathematics, he realised that an analogy must exist between the diffusion and Maxwell equations and strove to create diffusive counterparts to electromagnetism and optics:
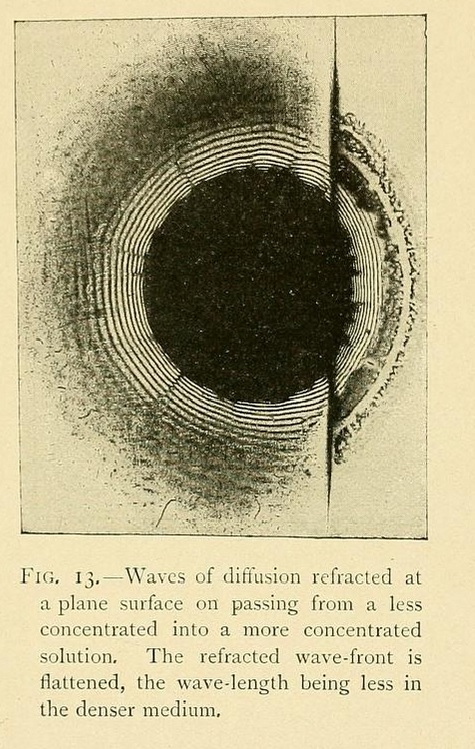
The rings in the figure are layers of silver arsenate precipitated when a drop of silver nitrate falls on a hot glass plate covered with a thin layer of gelatin solution containing sodium arsenate. Leduc extended these "optical" experiments to include the interference patterns created when a series of drops are "sown" in a straight line across the plate:
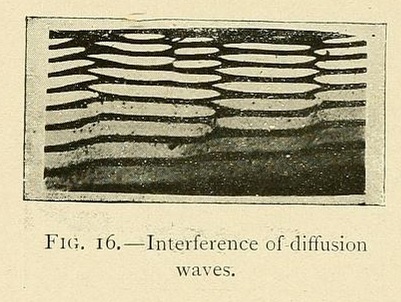
Leduc was perhaps a decade too early: had he worked a little later and known more mathematics he might have been reminded of interfering de Broglie waves! The free-particle Schrödinger equation is of course really just the diffusion equation generalised to the case of complex density and an imaginary diffusion coëfficient ih/4πm. Although the size and reality of Planck's constant mean that macroscopic diffusion waves and microscopic matter waves are not the same, the analogy between diffusive and quantum phenomena remains (in my opinion) both interesting and largely unexplored. In the 1960s, E. R. Fitzgerald of Johns Hopkins claimed to have discovered a class of quantum-like mesoscopic phenomena in crystalline solids governed by a particular finite-difference equation. Although Fitzgerald seems to have been unaware of it, this difference equation is in fact the discrete limit of Schrödinger's with a particular non-Planck value of h (a fact which, so far as I know, has never been mentioned in the literature before this blog posting).
Returning, however, to Leduc: the waves of diffusion led him to the study of periodic chemical reactions, a few of which were already known (although irrationally intense scepticism about them would persisist until the work of Belousov and Zhabotinsky in the 1950s); to crystallisation; and to the idea of the biological cell as a crystal-like unit precipitated from a solution. (He did not claim the idea was original, pointing out that it had been independently proposed by Moritz Traube, the founder of cell-membrane science, in 1866, and by the Euro-Texan philosopher Edmund Montgomery in 1867.)
Leduc believed that the Traube-Montgomery hypothesis, mostly ignored for thirty years, was the starting point of a new science, "synthetic biology". He meant this in the most literal sense, and attempted to synthesise biological organisms in the laboratory. Needless to say, he did not succeed at this, but he did produce astonishing "biomimetic" structures, what a geologist would call "pseudofossils". Surely, he argued, the emergence of such complex shapes from chemical and physical processes supports the likelihood that life itself, in its greater complexity, has a similar origin.
According to Clément, the reaction of the scientific community was hostile. One reason for this, mentioned by Leduc himself, was the still-recent controversy over spontaneous generation: if organisms could be precipitated from solution, what would become of Pasteur's germ theory? Clément proposes another reason: most biologists of the early 1900s supported some form of vitalism, and were inclined to reject any purely "physicalist" approach. (I suppose that this would have been particularly true in France, where Henri Bergson was a dominant influence on both science and the humanities.) I would add a third: pseudofossils, if truly indistinguishable from biological structures, would be a threat to historical palæontology, and thus to the theory of evolution itself, still controversial in 1911. Leduc (who was himself an evolutionist, albeit a Lamarckian) understood this, writing: "The palæontologist relies on the different forms found in his rocks to classify his specimens ; from the existence of a shell, he concludes the presence of life. Since, however, forms which are apparently organic may be merely the product of osmotic growth, it is evident that he must reconsider his conclusions." Given the dogmatic and polemic personalities of so many fin de siècle biologists, it is difficult to believe that such a reconsideration would have been eagerly welcomed.
OSMOTIC GROWTH:
A Study in Morphogenesis
Chapter XI of The Mechanism of Life by Dr. Stéphane Leduc (Professeur à l'École de Médecine de Nantes), tr. W. Deane Butcher. (New York: Rebman, 1911)
Leduc's words are in bold

The phenomenon of osmotic growth has doubtless presented itself to the eyes of every chemist ; but to discover a phenomenon it is not enough merely to have it under our eyes. Before Newton many a mathematician had seen a spectrum, if only in the rainbow ; many an observer before Franklin had watched the lightning. To discover a phenomenon is to understand it, to give it its due interpretation, and to comprehend the importance of the rôle which it plays in the scheme of nature.
Osmotic Membranes. --- Certain substances in concentrated solution have the property of forming osmotic membranes when they come in contact with other chemical solutions. When a soluble substance in concentrated solution is immersed in a liquid which forms with it a colloidal precipitate, its surface becomes encased in a thin layer of precipitate which gradually forms an osmotic membrane round it.
An osmotic membrane is not a semi-permeable membrane, as sometimes described, i.e. a membrane permeable to water but impermeable to the solute. It is a membrane which opposes different resistances to the passage of water and of the various substances in solution, being very permeable to water, but much less so to the different solutes.
A soluble substance thus surrounded by an osmotic membrane represents what Traube has called an artificial cell. In such a cell the dissolved substances have a very high osmotic pressure, an expansive force like that of steam in a boiler ; the molecules of the solute exerting pressure on the walls of the extensible cell, and distending it like the gas in a balloon. This pressure increases the volume of the cell, and in consequence water rushes in through the permeable membrane and still further distends the cell.
Most beautiful osmotic cells may be produced by dropping a fragment of fused calcium chloride into a saturated solution of potassium carbonate or tribasic potassium phosphate, the calcium chloride becoming surrounded by an osmotic membrane of calcium carbonate or calcium phosphate. This mineral membrane is beautifully transparent and perfectly extensible. It is astonishing to contemplate the contrast between the hard crystalline forms of ordinary chalk and these soft transparent elastic membranes which have the same chemical constitution. These osmotic cells of carbonate of lime or phosphate of lime consist of a transparent membrane enclosing liquid contents and a solid nucleus of chloride of calcium. Their form is that of an ovoid or flattened sphere, and they may attain a diameter of seven centimetres or more.
More frequently the osmotic growth consists of a number of cells instead of one large cell. The first cell gives birth to a second cell or vesicle, and this to a third, and so on, so that we finally obtain an association of microscopic cellular cavities, separated by osmotic walls -- a structure completely analogous to that which we meet with in a living organism.
We may easily picture to ourselves the mechanism by which an osmotic cell gives birth to such a colony of microscopic vesicles. The membranogenous substance, the chloride of calcium, diffuses uniformly on all sides from the solid nucleus, and forms an osmotic membrane where it comes into contact with the solution. This spherical membrane is extended by osmotic pressure, and grows gradually larger. Since the area of the surface of a sphere increases as the square of its radius, when the cell has grown to twice its original diameter, each square centimetre of the membrane will receive by diffusion but a quarter as much of the membranogenous substance. Hence, after a time, the membrane will not be sufficiently nourished by the membranogenous substance, it will break down, and an aperture will occur through which the interior liquid oozes out, forming in its turn a new membranous covering for itself. This is the explanation of the fact that all living organisms are formed by colonies of microscopical elements, although we must not forget that Nature often produces similar results in different ways.
Osmotic growths may be obtained from a great number of chemical substances. The most easily grown are the soluble salts of calcium in solutions of alkaline phosphates and carbonates, to which we have already alluded. We may also reverse the phenomenon by growing phosphates and carbonates in solutions of calcium salts, but in this case the osmotic growths are not so beautiful.
The various silicates play an important part in the constitution of shells and of the skeletons of marine animals. Most of the metallic salts, and more especially the soluble salts of calcium, give rise to the phenomenon of osmotic growth when sown in solutions of the alkaline silicates. In this way, by using different silicates and varying the proportions and the concentrations, we may obtain an immense variety of osmotic growths.
A good solution to commence with is the following : ---
Silicate of potash, sp. gr. 1.3 (33° Beaumé): 60 gr.
Saturated solution of sodium carbonate: 60 gr.
Saturated solution of dibasic sodium phosphate: 30 gr.
Distilled water: make up to 1 litre.
A fragment of fused calcium chloride dropped into this solution will produce a rapid growth of slender osmotic forms which may attain a height of 20 or 50 centimetres.
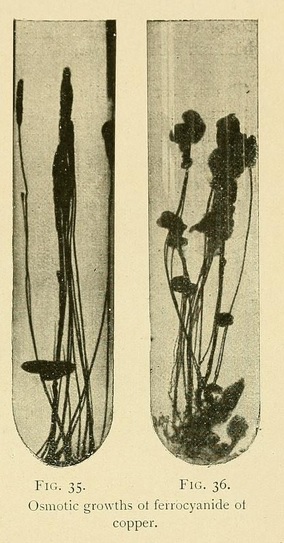
Small pellets may also be made of one part of sugar and two of copper sulphate and sown in the following solution, which must be kept warm until the growth is complete : ---
Ten per cent, solution of gelatine: 10 to 20 c.c.
Saturated solution of potassium ferrocyanide: 5 to 10 c.c.
Saturated solution of sodium chloride: 5 to 10 c.c.
Warm water (32° to 40°C.): 100 c.c.
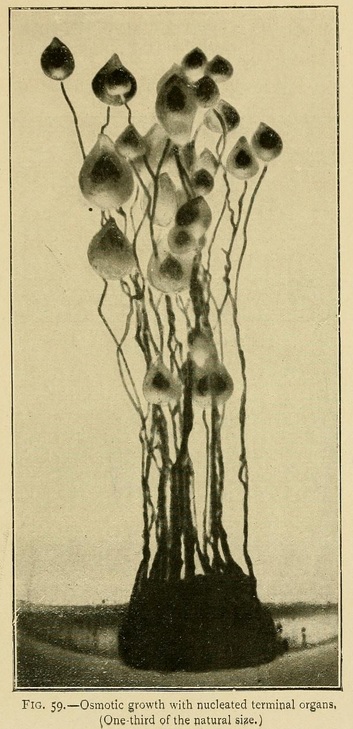
In this solution we can obtain osmotic growths which may attain to a height of 40 centimetres or more, vegetable forms, roots, arborescent twigs, leaves, and terminal organs. These growths are stable as soon as the gelatine has cooled and set, and may be carried about without fear of injury (Fig. 35).
Precipitated osmotic membranes are very widely distributed in nature. Professor Ulenhuth has seen iron growths in alkaline sodium hypochlorite (Javelle water), and Lecha-Marzo has demonstrated the osmotic growth of the various stains used for microscopy, in the liquids used for fixing preparations.
We now know that the physical force which builds up these growths is that of osmotic pressure, since the slightest consideration will show the inadequacy of the usual explanation that the growth is due to mere differences of density, or to amorphous precipitation around bubbles of gas. These may indeed affect the phenomenon, but can in no way be regarded as its cause.
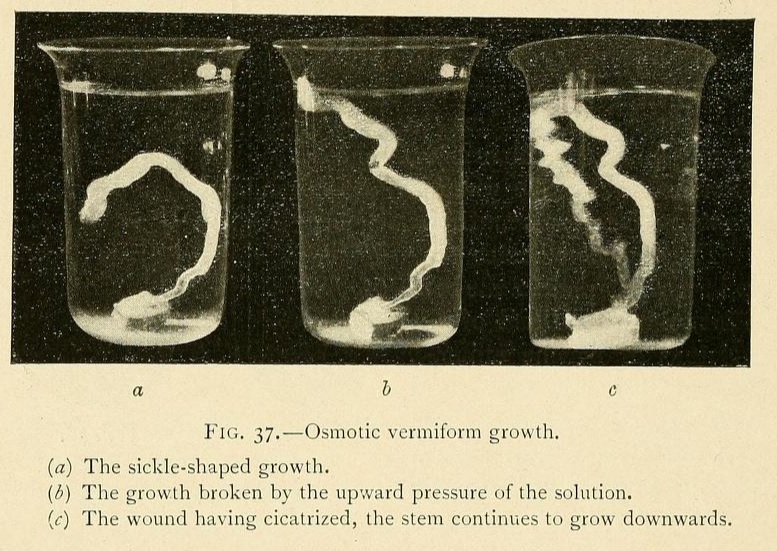
One of our experiments throws considerable light on this question. In a glass vessel we placed a concentrated solution of carbonate of potassium, to which had been added 4 per cent. of a saturated solution of tribasic potassium phosphate. Into this solution we dropped a fragment of fused calcium chloride, and obtained a vermiform growth some 6 millimetres in diameter. This growth was curved, at first growing upwards, then for a short distance horizontally, and finally downwards. The upward pressure of the solution, which was heavier than the growth, ultimately broke it at the top of the curve, as shown at b, Fig. 37. The liquid contents of the growth began to ooze out through the wound, but this after a time became cicatrized, and the stem continued to grow obstinately downwards once more, in opposition to the hydrostatic pressure. In consequence of this pressure the growth is sinuous, tacking as it were from side to side like a boat against the wind. We give three successive photographs of this growth, which attained a length of over 10 inches. We have frequently obtained these vermiform growths forming a series of such loops, growing upwards and falling again many times in succession.
Osmotic Growths in Air. --- Certain of these artificial cells may be made to grow out of the solution into the air. For this purpose we place a fragment of CaCl2 in a shallow flat-bottomed glass dish, just covering the fragment with liquid. The best solution is as follows : ---
Potassium carbonate, saturated solution: 76 parts.
Sodium sulphate, saturated solution: 20 parts.
Tribasic potassium phosphate, saturated solution: 4 parts.
The calcium chloride surrounds itself with an osmotic membrane ; water penetrates into the interior of the cell thus formed, and a beautiful transparent spherical cell is the result, the summit of which soon emerges from the shallow liquid. The cell continues to increase by absorption of the liquid at its base, and may grow up out of the liquid into the air for as much as one or two centimetres.
This is a most impressive spectacle, an osmotic production, half aquatic and half aerial, absorbing water and salts by its base, and losing water and volatile products by evaporation from its summit, while at the same time it absorbs and dissolves the gases of the atmosphere.
The aerial portion of an osmotic growth will sometimes become specialized in form. The summit of the growth develops a sort of crown or cup surrounded by a circular wall. This cup contains liquid, and continues to grow up into the air like the stem of a plant, carrying with it the liquid which has been absorbed by the base of the growth.
The preceding experiments give us an explanation of the curious phenomena exhibited by so-called creeping salts. A saline solution left at the bottom of a vessel will sometimes be found after some months to have crept up to the top of the vessel. Cellular partitions formed in this way will be found extending from the bottom to the top of the vessel, and not only so, but the whole of the remaining liquid will be imprisoned in the upper cells.
Assimilation and Excretion. --- Like a living being, an osmotic growth absorbs nutriment from the medium in which it grows, and this nutriment it assimilates and organizes. If we compare the weight of an osmotic growth with that of the mineral fragment which produced it, we shall find that the mineral seed has increased many hundred times in weight. Similarly, if we weigh the liquid before and after the experiment, we shall find that it has lost an equivalent weight. The absorbed substance of an osmotic production must also undergo chemical transformation before it can be assimilated -- that is, before it can form part of the growth. Calcium chloride, for example, growing in a solution of potassium carbonate, is transformed into calcium carbonate.
CaCl2 + K2CO3 = CaCO3 + 2KCl.
Thus an osmotic growth can make a choice between the substances offered to it, rejecting the potassium of the nutrient liquid, and absorbing water and the radical CO3, while at the same time it eliminates and excretes chlorine, which may he found in the nutrient liquid after the reaction.
Of all the ordinary physical forces, osmotic pressure and osmosis alone appear to possess this remarkable power of organization and morphogenesis. It is a matter of surprise that this peculiar faculty has hitherto remained almost unsuspected.
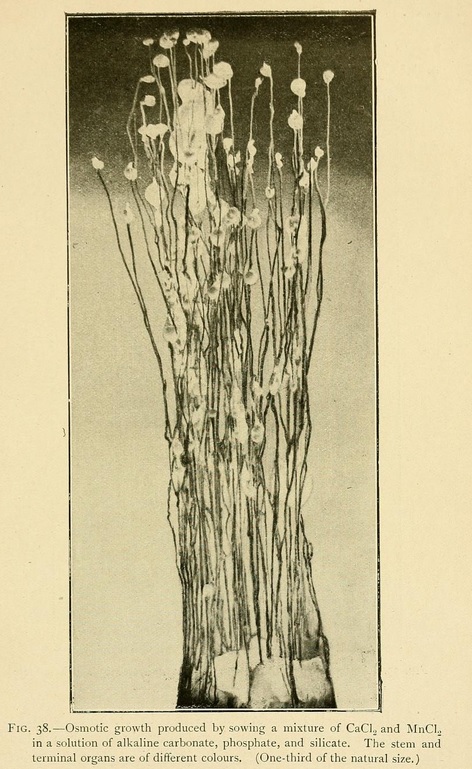
Osmotic Growths. --- If we sow fragments of calcium chloride in solutions of the alkaline carbonates, phosphates, or silicates, we obtain a wonderful variety of filiform and linear growths which may attain to a 30 or 40 centimetres. Some are so flexible that the stems bend, falling in curves around the centre of growth, like leaves of grass. If we dilute this same liquid, as it becomes less concentrated the growths are more curved, ramified, dendritic, like those of trees or corals.
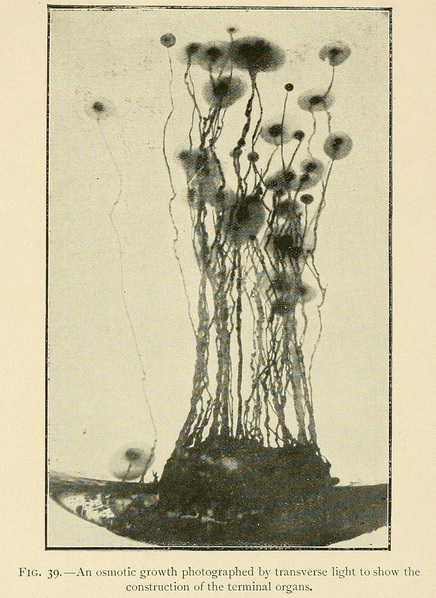
In the culture of osmotic growths we may also by appropriate means produce terminal organs resembling flowers and seed-capsules. To do this we wait till the growth is considerably advanced, and then add a large quantity of liquid to the nutrient solution so as to diminish the concentration a hundredfold or more. Spherical terminal organs will then grow out from the ends of the stems, which may during their further growth become conical or piriform in shape.
By superposing layers of liquid of different concentration and decreasing density, one may obtain knots and swellings in the osmotic growths marking the surfaces of separation of the liquid. When a young growth in the vigour of its youth reaches the surface of the water, it spreads out horizontally over the surface of the liquid in thin leaves or foliaceous expansions of different forms.
The preponderating influence in morphogenesis is osmotic pressure, the osmotic forms varying with its intensity, distribution, and mode of application. Whatever the chemical composition of the liquid, similar osmotic forces, modified in the same manner, give rise to forms which have a family resemblance. The chemical nature of the liquid, however, is not entirely without influence on the form. Thus the presence of a nitrate in the mother liquor tends to produce points or thorns:

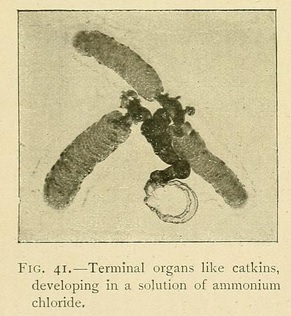
Ammonium chloride in a potassium ferrocyanide solution produces growths shaped like catkins, and the alkaline chlorides tend to produce vermiform growths.
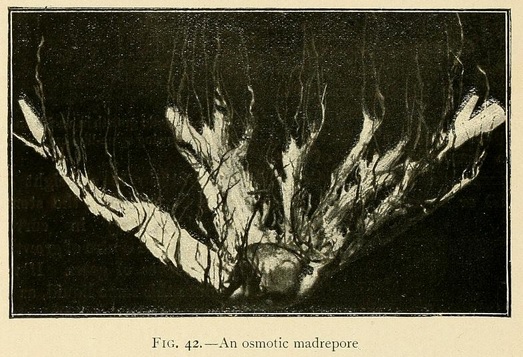
Coralline growths may also be obtained by using appropriate chemical solutions. For this purpose the solution of silicate, carbonate, and dibasic phosphate should be diluted to half strength, with the addition of 2 to 4 per cent. of a concentrated solution of sodium sulphate or potassium nitrate. Coral-like forms may also be grown from a semi-saturated solution of silicate, carbonate, and dibasic phosphate, to which has been added 4 per cent. of a concentrated solution of sodium sulphate or potassium nitrate. In this we may obtain beautiful growths like madrepores or corals, formed by a central nucleus from which radiate large leaves like the petals of a flower.
The presence of nitrate of potassium produces pointed leaves with thorn-like processes recalling the forms of the aloe and the agave.
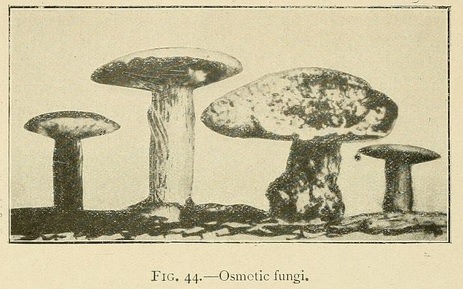
Most remarkable fungus-like forms may be obtained by commencing the growth in a concentrated solution, and then carefully pouring a laver of distilled water over the surface of the liquid. The resemblance is so perfect that some of our productions have been taken for fungi even by experts.
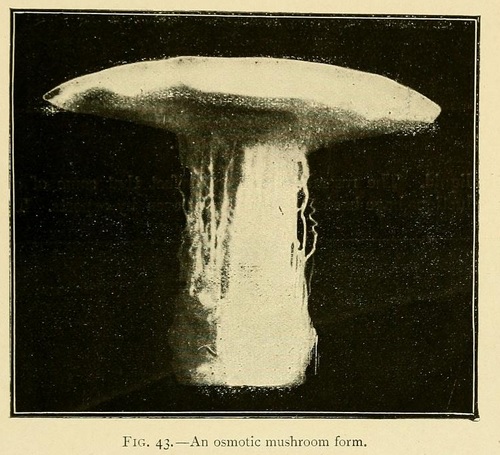
The stem of these osmotic fungi is formed of bundles of fine hollow fibres, while the upper surface of the cap is sometimes smooth, and sometimes covered with small scales. The lower surface of the cap shows traces of radiating lamellæ, which are sometimes intersected by concentric layers parallel to the outer surface of the cap. In this case the lower surface of the cap shows a number of orifices or canals similar to those seen in many varieties of fungus.
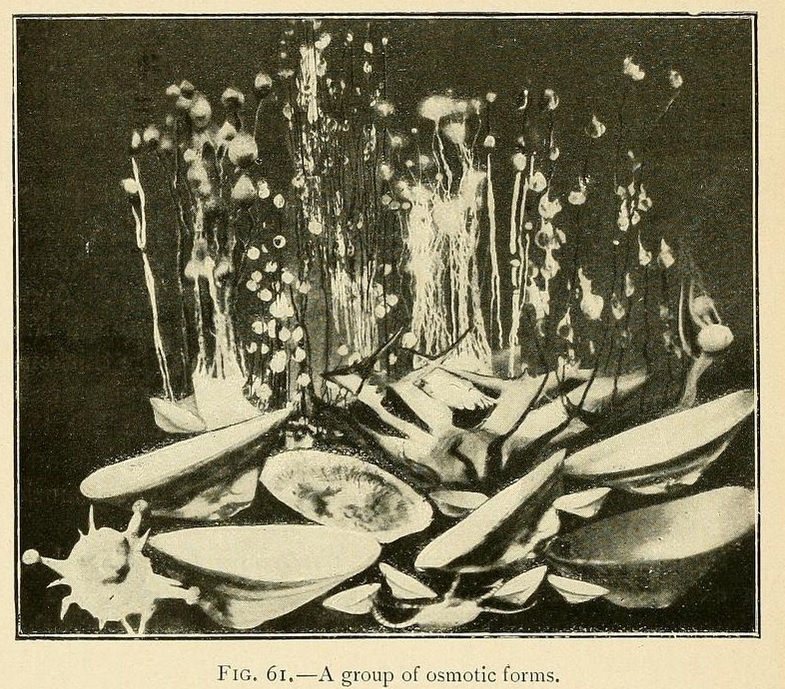
Shell-like osmotic productions may be grown by sowing the mineral in a very shallow layer of concentrated solution, a centimetre or less in depth, and pouring over this a less-concentrated layer of solution.

By varying the solution or concentration we may thus grow an infinite variety of shell forms:
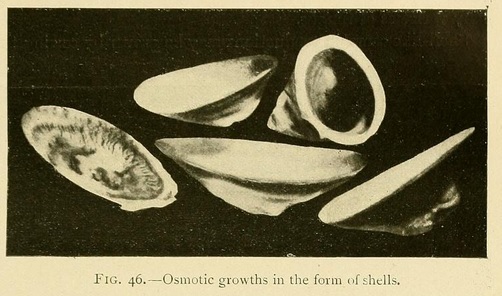
Capsules or closed shells may be produced in the same way by superimposing a layer of somewhat greater concentration. These capsules consist of two valves joined together at their circumference. The lower valve is thick and strong, while the upper valve may be transparent, translucent, or opaque, but is always thinner and more fragile than the lower one.
Ferrous sulphate sown in a silicate solution gives rise to growths which are green in colour, climbing, or herbaceous, twining in spirals round the larger and more solid calcareous growths.
With salts of manganese, the chloride, citrate or sulphate, the stages of evolution of the growth are distinguished not only by diversities of form, but also by modifications of colour. We may thus obtain terminal organs black or golden yellow in colour on a white stalk.

In a similar way we may obtain fungi with a white stalk and a yellow cap, of which the lower surface is black.
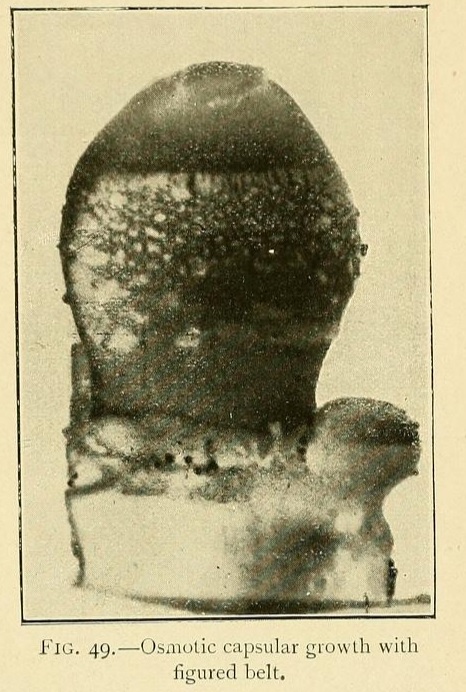
Very beautiful growths may be obtained by sowing calcium chloride in a solution of potassium carbonate, with the addition of 2 per cent. of a saturated solution of tribasic potassium phosphate. This will give capsules with figured belts, vertical lines at regular intervals, or transverse stripes composed of projecting dots such as may be seen in many sea-urchins.
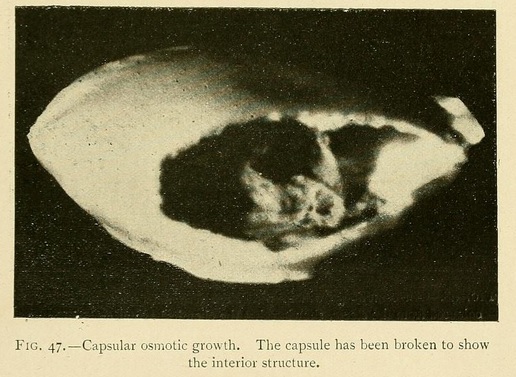
These capsules are closed at the summit by a cap, forming an operculum, so that they sometimes appear as if formed of two valves. Now and again we may see the upper valve raised by the internal osmotic pressure, showing the gelatinous contents through the opening.
The calcareous capsules grown in a saturated solution of potassium carbonate or phosphate often take a regular ovoid form. If these are allowed to thicken, they may be taken out of the water without breaking, and then present the aspect of veritable ooliths.
Osmotic productions may be divided into two groups. Some like the silicate growths are fixed. Like vegetables, they develop, become organized, grow, decline, die, and are disintegrated at the spot where they are sown.
Others, especially those which are grown in alkaline carbonates and phosphates, have two periods of evolution, the first a fixed period, and the second a wandering one. During the first period their specific gravity is greater than that of the surrounding medium and they rest immobile at the bottom of the vessel in which they are sown. As they grow, they absorb water and their specific gravity diminishes.
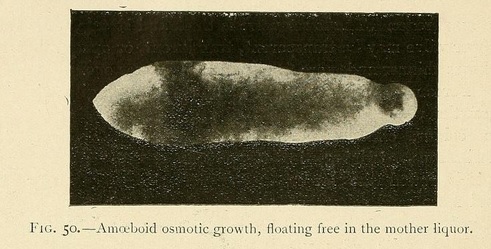
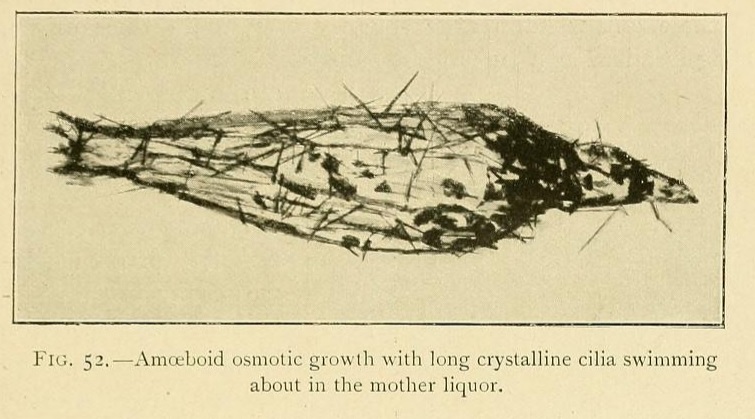
Little by little they rise up in the liquid, and finally acquire a considerable amount of mobility, being readily displaced by every current. Hence it is very difficult to photograph these mobile osmotic growths, which swim about in the mother hquor and are often provided with prolongations in the forms of cilia, and sometimes with fins, which undulate as they move.
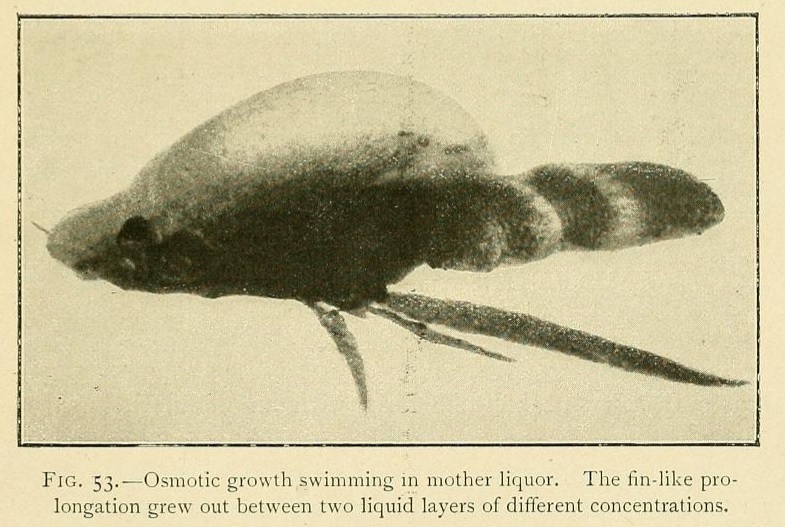
Some of these ciliary hairs are evidently osmotic in their origin, being localized as a tuft at the summit of the growth. Others are apparently crystalline in structure, and are spread over the whole surface of the swimming vesicle. An osmotic growth increases by the absorption of water from a concentrated solution. When the solution is originally saturated it thus becomes supersaturated, and deposits these long ciliary crystals on the surface of the growth.
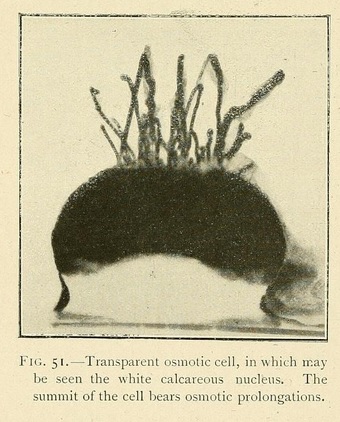
When a capsule splits in two under the influence of the internal osmotic pressure, it may happen that the operculum or upper valve floats away in the liquid. We thus obtain a free swimming organism, a transparent bell-like form with an undulating fringe, like a Medusa.
Frequently a single seed or stock will give rise to a whole series of osmotic growths. A vesicle is first produced, and then a contraction appears around the vesicle, and this contraction increases till a portion of the vesicle is cut off and swims away free like an amoeba. The same phenomenon may be observed with vermiform growths, a single seed often giving rise in this way to a whole series of amoebiform or vermiform productions.
It must be remembered that in an osmotic growth the active growing portion is the gelatinous contents in the interior, the external visible growth being only a skeleton or shell. We may sometimes succeed in hooking up one of these long vermiform growths, breaking the calcareous sheath, and drawing out a long undulating translucid gelatinous cylinder. The outline of this cylinder is so well defined as to make us doubt whether the fine colloidal membrane which separates it clearly from the liquid can have been formed so rapidly, or if it may not perhaps exist already formed in the interior of its calcareous sheath.
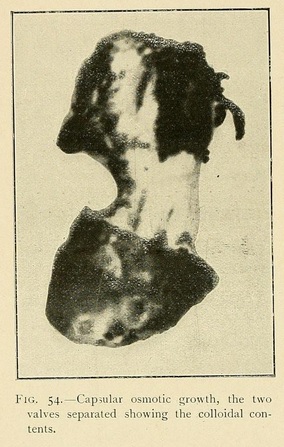
When a large capsular shell such as we have described bursts, it expels a part or the whole of its contents as a gelatinous mass which retains the form of the cavity. Similarly, if we suddenly dilute the mother liquor around an osmotic cell, it bursts by a process of dehiscence, and projects into the liquid a part of its contents, which may thus become an independent vesicle. In this way a single osmotic cell may produce a whole series of independent vesicles.
It is even possible to rejuvenate an osmotic growth that has become degenerate through age. An osmotic production grows old and dies when it has expended the osmotic force contained in the interior of its capsule. A calcium osmotic growth which has thus become exhausted may be rejuvenated by transferring it to a concentrated solution of calcium chloride. It will absorb this, and thus be enabled to renew its evolution and growth when put back again into the original mother liquor.
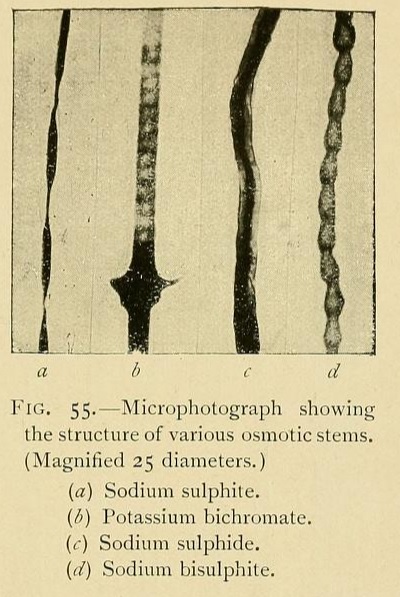
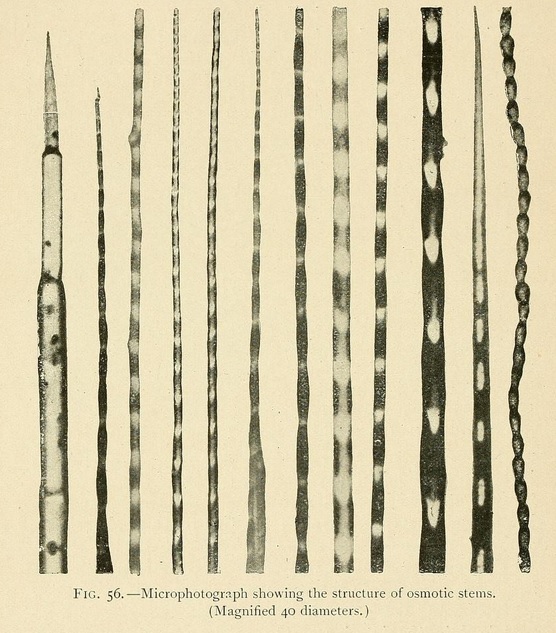
The structure of osmotic growths is no less varied than their form. Their stems are formed of cells or vesicles juxtaposed, showing cavities, separated by osmotic walls. Sometimes the component vesicles have kept their original form, so that the stem has the appearance of a row of beads. Or the cells may be more or less flattened, the divisions being widely separated. Or again, by the absorption of the divisions, a tube may be formed, a veritable vessel or canal in which liquids can circulate.
The foliaceous expansions, or osmotic leaves, also present great varieties both of appearance and of structure.
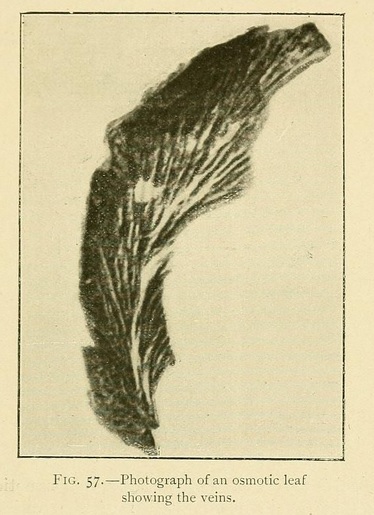
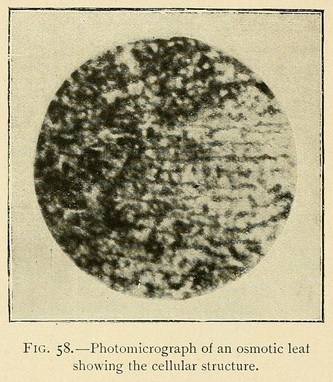
The veins may be longitudinal, fan-shaped, or penniform. We have occasionally met with leaves having a lined or ruled surface, giving most beautiful diffraction colours. The usual structure, however, is vesicular or cellular, as in Fig. 58:
In conclusion we may say that osmotic growths are formed of an ensemble of closed cavities of various forms, containing liquids and separated by osmotic membranes, constituting veritable tissues. This structure offers the closest resemblance to that of living organisms.
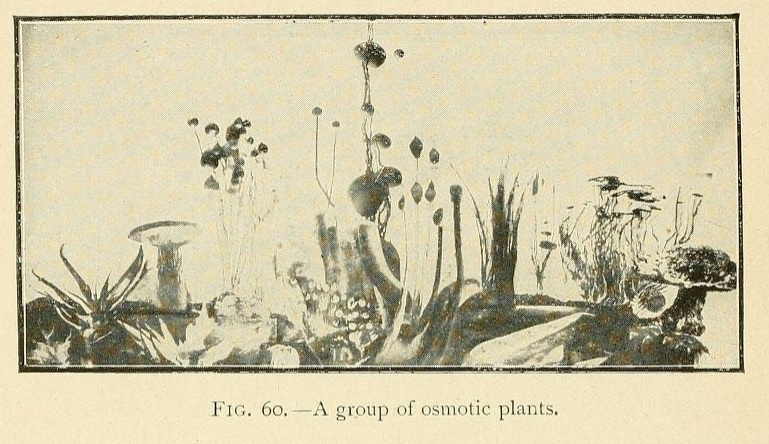
Is it possible to doubt that the simple conditions which produce an osmotic growth have frequently been realized during the past ages of the earth ? What part has osmotic growth played in the evolution of living forms, and what traces of its action may we hope to find to-day ? Osmotic growth gives us fibrous silicates, phosphatic nodules, corals, and madrepores ; it also gives us formations which remind one of the "atolls," calcareous growths rising like a crown out of the water.
The geologist may well consider what rôle osmotic growth may have played in the formation of the various rocks, siliceous, calcareous, barytic, magnesian, the fibrous and nodular rocks and atolls. The palaeontologist relies on the different forms found in his rocks to classify his specimens ; from the existence of a shell, he concludes the presence of life. Since, however, forms which are apparently organic may be merely the product of osmotic growth, it is evident that he must reconsider his conclusions. The same may be said of the various forms of coral or of fungoid growths. In the presence of a calcified or silicated fungus we can no longer argue with certainty as to the existence of life, without taking into consideration the possibility that the specimen in question may be an osmotic production.
Whatever our opinion as to its signification, osmotic growth demands the attention of every mind devoted to the study of nature. It is a marvellous spectacle to see a formless fragment of calcium salt grow into a shell, a madrepore, or a fungus, and this as the result of a simple physical force. Why should the study of osmotic growth attract less attention than the formation of crystals, on which so much time and labour has been bestowed in the past ?