Michael L. Fine
Department of Biology
Virginia Commonwealth University
Richmond, VA 23284-2012
We (Fine and collaborators) have studied various aspects of acoustic communication (sound production and hearing) in the oyster toadfish, channel catfish and several sciaenid species. Highlights include:
- Neuroanatomy and gross anatomy of sonic structures and pathways
- Sexual dimorphism of the brain, sonic muscle and swimbladder
- Electrical stimulation of the brain to evoke agonistic and mating calls
- Identifying sonic and steroid-concentrating neurons
- Hormonal (steroid) basis of sexual dimorphism
- Neuromuscular development
- Sonic muscle ultrastructure and physiology
- Call generation and variation
- Identification of individual toadfish in field recordings
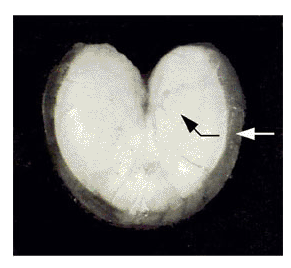
Our current and future emphasis will focus on understanding mechanisms of sound production, acoustic radiation and propagation of fish sounds. This information will aid in interpreting data from passive acoustics. In some species, for instance, sound frequency varies with fish size, but in others it is size independent. We have demonstrated that the toadfish advertisement call has a mildly directional pattern (several dB greater behind the fish), which correlates with its unusual heart shape (Fig. 1). Depending on the radiation of sounds from sciaenid fishes, their amplitude (a function of fish size and distance) might also vary with the fish’s profile relative to the hydrophone.
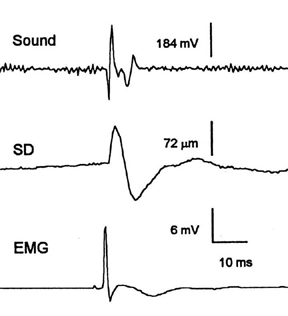
Historically, the fish swimbladder has been characterized as a pulsating, resonant underwater bubble, which provides an omnidirectional (monopole) acoustical source useful in sound production and hearing. We contend that the classic beliefs about swimbladder acoustic function may not apply to all fishes. Fish sonic muscles are the fastest in vertebrates, something that does not seem necessary for exciting a resonant structure, i.e. ringing a bell or tapping a crystal wine glass. Recent work on the toadfish sonic muscle-swimbladder system provides evidence against the classic conceptions: its radiation pattern is directional, damping is rapid (Fig. 2), equivalent to an automobile shock absorber, a device that inhibits resonance, and sonic muscles drive it in an inefficient quadrupole fashion (Fig. 1) rather than as a pulsating monopole. Examination of swimbladder displacement, velocity and acceleration indicates that peak acoustic pressure occurs at the velocity maximum (Fig. 3). Therefore, rapid movement of the bladder appears to be necessary to generate an audible sound. Finally, deflating the bladder does not affect the toadfish audiogram (see Hong Yan), arguing against its classic role in audition.
It is our goal to provide similar information on species of interest for passive acoustics to aid in interpretation of calls recorded in the field.
 |
 |
|
| Fig. 1. Toadfish swimbladder illustrating heart shape and lateral position of sonic muscles. Muscle contraction pushes sides in (white arrow) which pushes bottom out (black arrow). | Fig. 2. Electrically-stimulated sonic muscle twitch and resulting muscle action potential (EMG), swimbladder displacement (SD), and evoked sound. Note rapid damping of swimbladder displacement and sound termination as bladder movement continues, i.e. only rapid movement generates audible sound. |
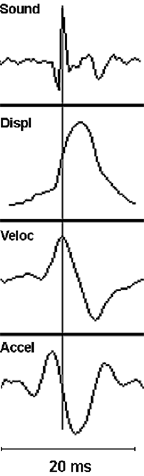 |
| Fig. 3. Sound (top panel) and swimbladder motion (displacement, velocity and acceleration caused by a single muscle twitch. Note that the peak of sound pressure occurs at the velocity maximum. |
Return to Top | Research Programs