|
Archives:
Summer/Fall 2001 Table
of Contents Balancing budgets is not exactly what you’d expect to find a marine geochemist doing, but it’s key to Matt Charette’s current research on coastal ponds. Charette, an assistant scientist and Coastal Ocean Institute Fellow at the Woods Hole Oceanographic Institution, is using a chemical tracer–radium isotopes–to look at sub-surface groundwater pathways to coastal embayments in southern New England. These pathways, also known as submarine groundwater discharge (SGWD), are thought to play a role in delivering nutrients, such as nitrate and phosphate, to coastal waters. "Typically," explains Charette, "there are three ways to measure how much SGWD is coming into an estuary or coastal ocean. You can create a water budget, manually measure the groundwater by using seepage meters, or you can use chemical tracers, which have become popular in the last decade." These methods are not without limitations, however.
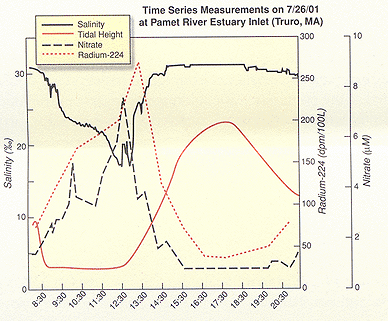
As one might expect, each chemical tracer has its advantages and disadvantages. "Radium is the tool we’re using for determining how much submarine groundwater is coming in," says Charette, whose study sites for the tracer experiments are Quonochontaug ("Quonny") Pond in Rhode Island, Sippewissett Marsh and Pamet River estuary, both on Cape Cod. Both Quonny Pond and Sippewissett Marsh represent what Charette calls "urbanized coastal sites," with Pamet serving as his control site. The field portion of his research involves two separate sampling protocols: one for groundwater, using residential wells or piezometers to collect samples; the other for the estuary, using a multi-probe device that measures salinity, dissolved oxygen, depth, pH, and tidal height. Groundwater sampling takes place on a different day than estuary sampling and, says Charette, the rule of thumb is to collect as many groundwater samples as possible. "Determining the average concentration of the tracer in groundwater is the main source of error in the whole approach. The groundwater content of radium can be variable, so with more samples, the average becomes more representative of the local conditions." The radium isotope work takes place back in the laboratory, using water samples collected in the field. Charette is assisted in the field and in the lab by Craig Herbold, a research assistant in the WHOI Marine Chemistry and Geoechemistry department. In addition to WHOI Sea Grant support, the work is funded by The Cove Point Foundation. Sampling in different seasons is important, as is sampling over the course of a tidal cycle. Charette sets up his instrumentation at the ocean inlet to "get a good look at nutrient fluxes" between the water coming into and out of the estuary. "In March, what came in to Quonny Pond, came out. There isn’t much biological activity in the winter." That was quite different, says Charette, than sampling in May and August, where he saw negative values for nutrients, also known as a nutrient "sink." This came as a surprise. "Quonny Pond is actually a sink for nutrients, meaning that nutrients are coming into the pond from Long Island Sound, versus traveling to the sound via the pond. Those results make it difficult to assess if there is a nitrogen source to Quonny Pond from groundwater. At this point, if there is, it’s minimal," says Charette. An altogether different kind of surprise presented itself in Pamet. "Pamet was supposed to be our pristine control site," begins Charette. "We thought it would be the least impacted of the three sites. As it turns out, it may be the most impacted." Charette describes a sampling trip to Pamet in July, when he saw a rapid increase in nitrate concentration as the tide went out, over two tidal cycles. "It peaked at 8 ÁM/l (micro-moles per liter)." As a basis for comparison, the nitrate concentration in Cape Cod Bay, the body of water that the Pamet estuary empties into, was less than 0.01 ÁM/liter.
Charette says that if he backtracks the nitrate peak to zero salinity, the spike he saw in Pamet corresponds with the nitrate concentration he’s seeing in the groundwater. For one thing, says Charette, there is not a lot of surface runoff to Pamet. That, coupled with the results of groundwater samples taken from wells along the fringe of the estuary, seems to implicate groundwater as the primary source of nitrate. As for the surprises, Charette seems more intrigued than disappointed. "Typically, the higher the housing density around an estuary, the higher the impact from groundwater." (Housing density around Pamet, compared to the other sites, is much lower.) "We definitely didn’t expect the nitrate signal to be as distinct as it was in Pamet." As to why, Charette can’t give a definitive answer, but guesses that older homes, many with cesspools as opposed to newer, Title V septic systems–and many located right at the edge of the estuary, have something to do with the strong nitrate signal. "You need time and distance for septic systems to dissipate the nitrate signal," he says, and both are missing in the Pamet equation. Now that he can determine if groundwater is delivering nutrients to an estuary, and how much nitrogen, in the form of ammonium and nitrate, is getting into the estuary through groundwater, Charette is gearing up to answer perhaps the most important question: what is the source of the groundwater-borne nitrogen? To help answer that question, Charette will work
with colleagues at the Boston University Marine Program, professor
Ivan Valiela and graduate student Kevin Kroeger, using stable nitrogen
isotopes. Possible culprits include wastewater and fertilizer, not
surprising in developed areas. However, being able to identify trouble
spots–and sources–will be useful to resource managers
constantly plagued by the effects of too much nitrogen in coastal
embayments. |